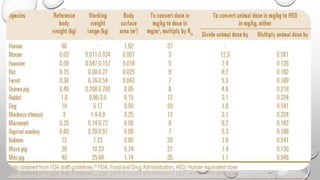
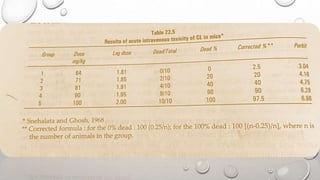
This document summarizes the objectives and methods of toxicity studies. The key points are:
1) Toxicity studies determine the lethal dose and therapeutic index of new drugs, and detect acute, chronic, genotoxic, and carcinogenic effects through tests in animals.
2) Studies must comply with good laboratory practice and be approved by ethics committees. Acute, subacute, and chronic toxicity tests are used to identify target organs and no observed adverse effect levels.
3) Acute toxicity tests determine the median lethal dose through single dose studies in mice and other species. Subacute and chronic tests involve daily dosing over weeks or months to identify pathological changes. Special tests evaluate effects on fertility, teratogenic



![BIOMEDICAL ETHICS
• BEFORE CONDUCTING ANY TOXICOLOGICAL STUDY OR TAKING ANY
TISSUE/CELLS FROM THE ANIMALS, THE STUDY SHOULD BE APPROVED BY
INSTITUTE ANIMAL ETHICS COMMITTEE (IAES) OR SHOULD SUBSCRIBE TO THE
LOCAL GUIDELINES
[ US FDA, EMA, CPCSEA ]
IN INDIA WE HAVE THE COMMITTEE FOR THE PURPOSE OF CONTROL AND
SUPERVISION OF EXPERIMENTS ON ANIMALS [CPCSEA]](https://image.slidesharecdn.com/toxicitystudy-210422074313/85/Toxicity-study-4-320.jpg)