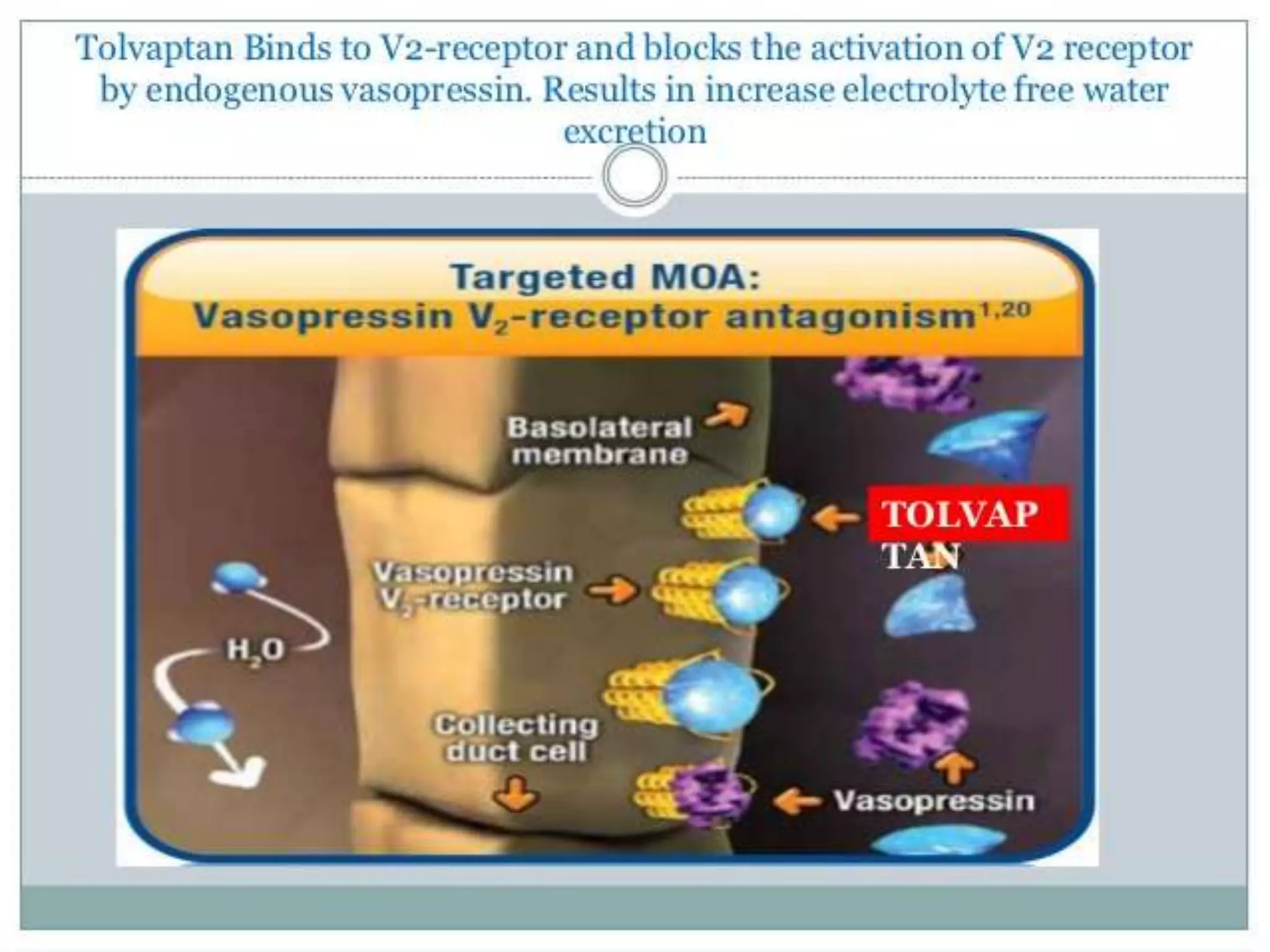
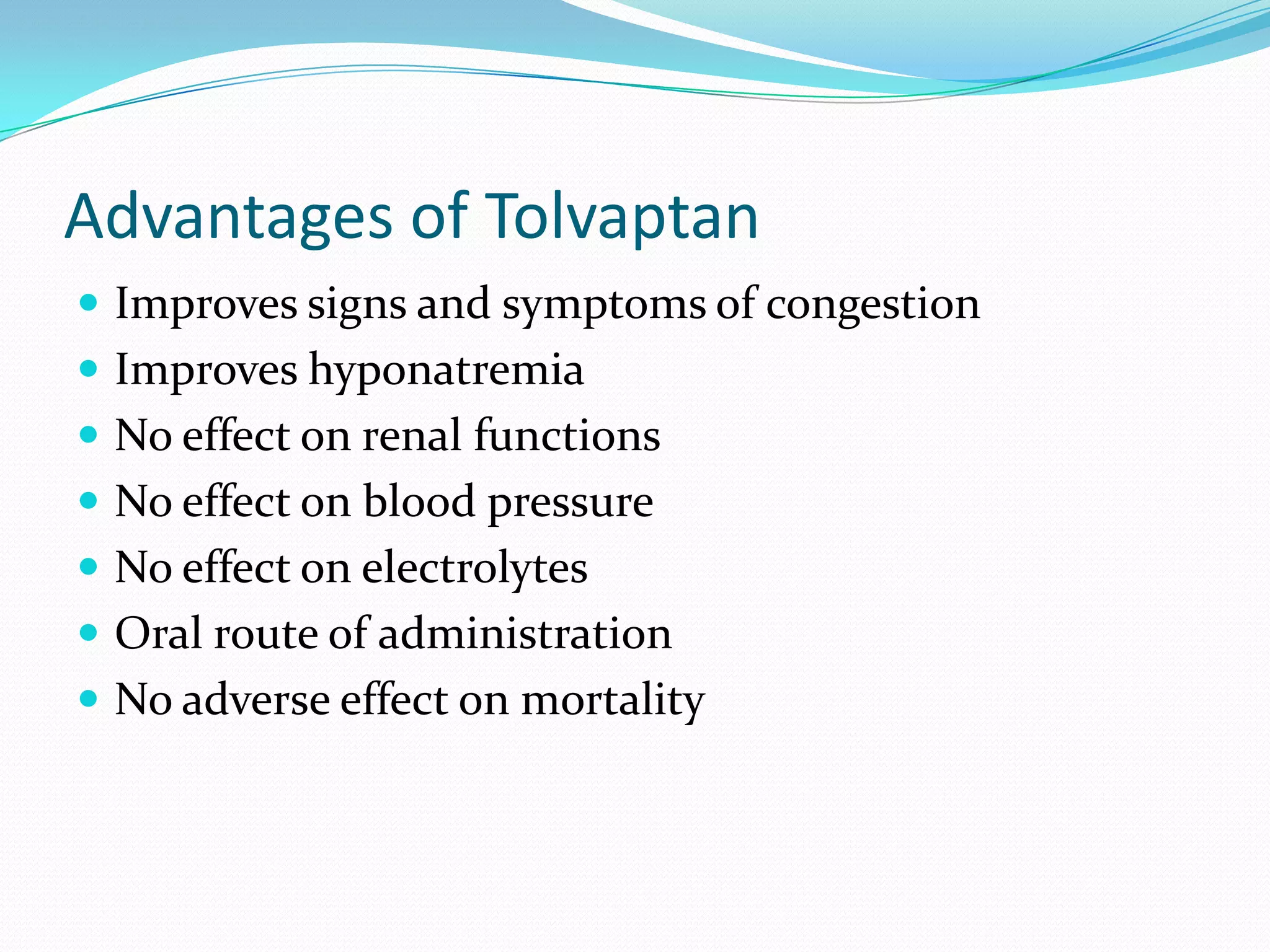
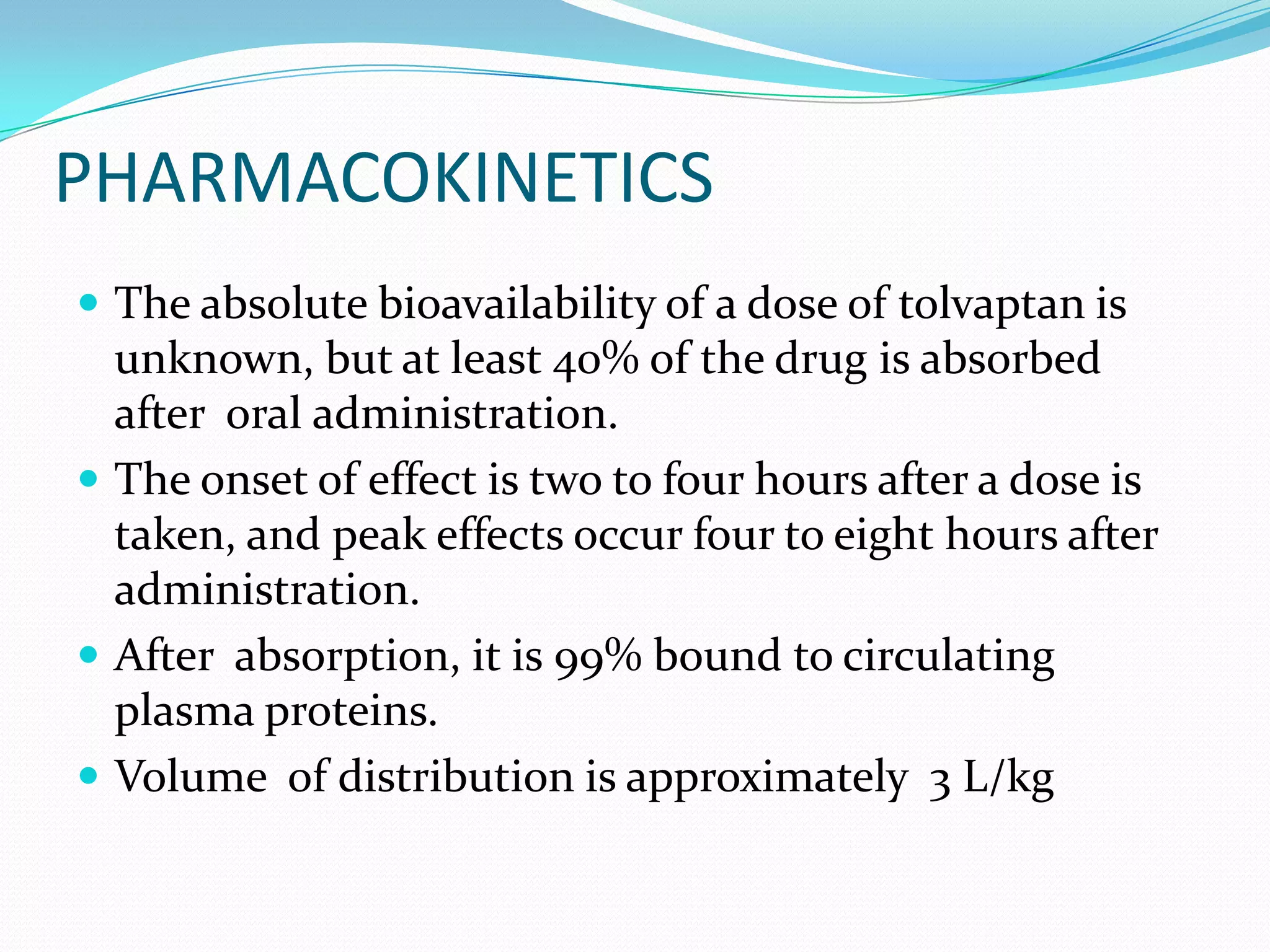
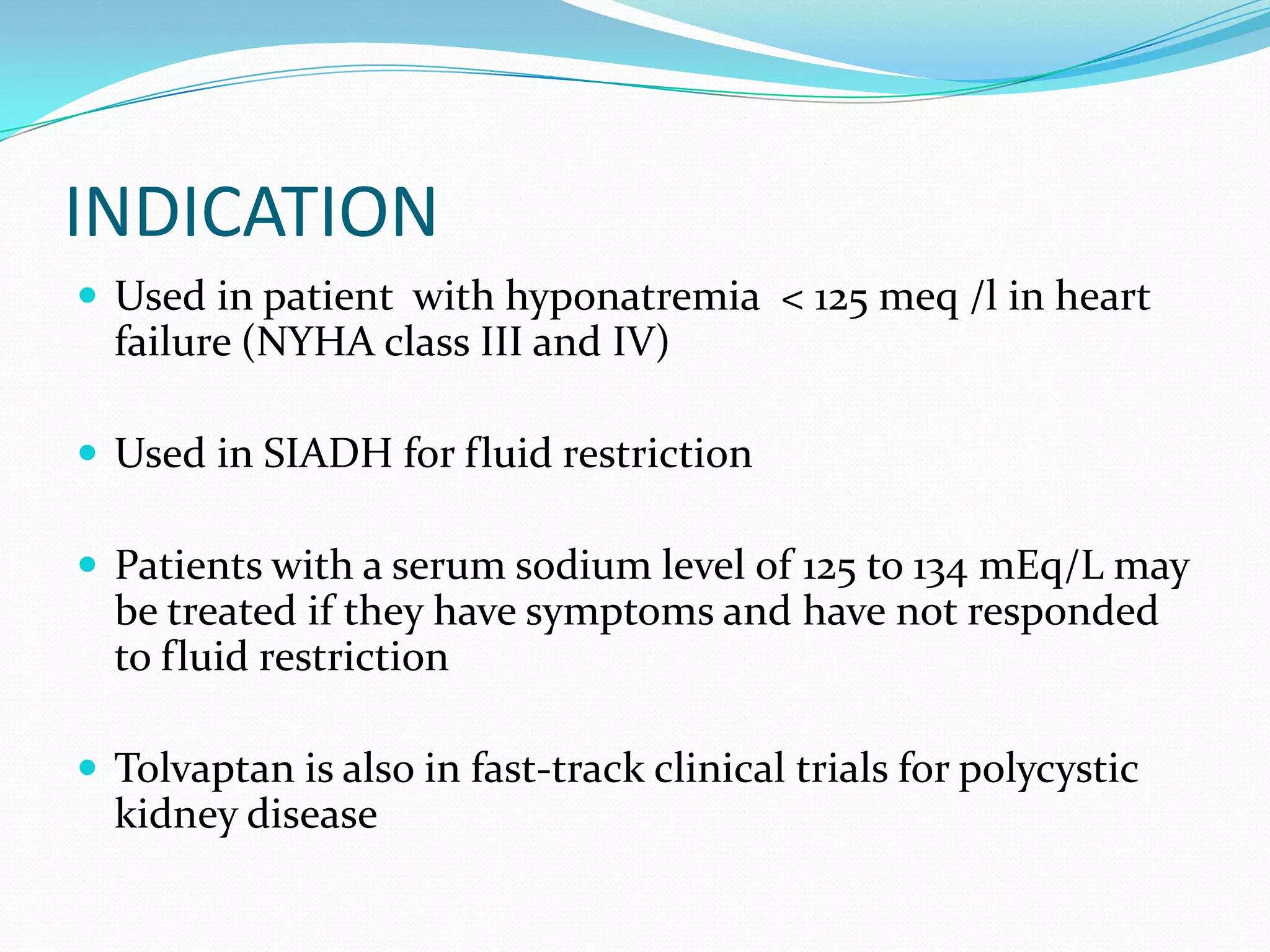
Tolvaptan is a selective vasopressin V2 receptor antagonist used to treat hyponatremia. It works by blocking V2 receptors in the kidney collecting ducts, decreasing water reabsorption and increasing excretion to raise serum sodium levels. Tolvaptan is approved by the FDA to treat hyponatremia caused by congestive heart failure, cirrhosis, or SIADH. It has few side effects but can cause thirst, dry mouth, and polyuria. Care must be taken to avoid overly rapid correction of sodium levels which can cause serious neurological issues.