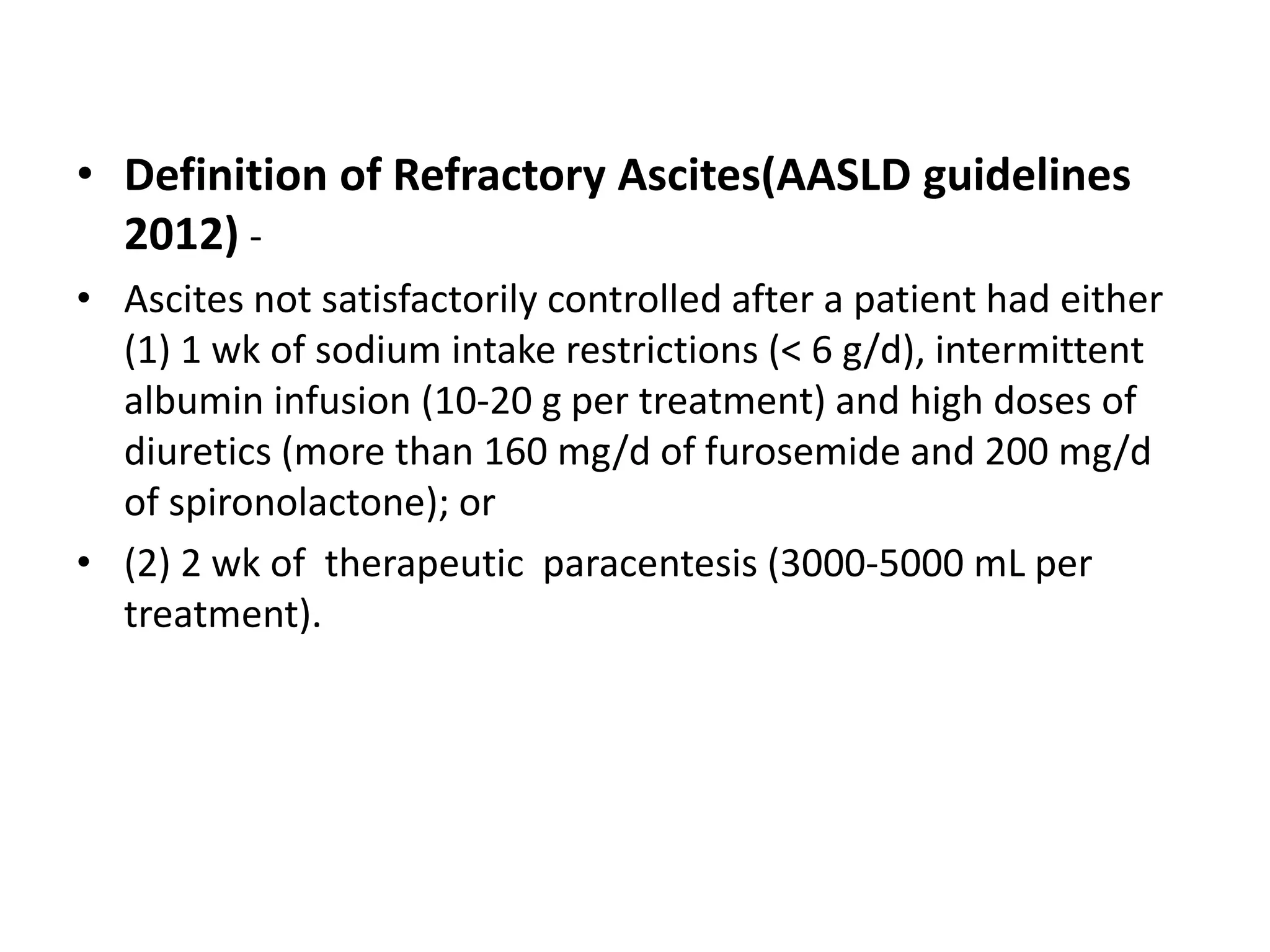
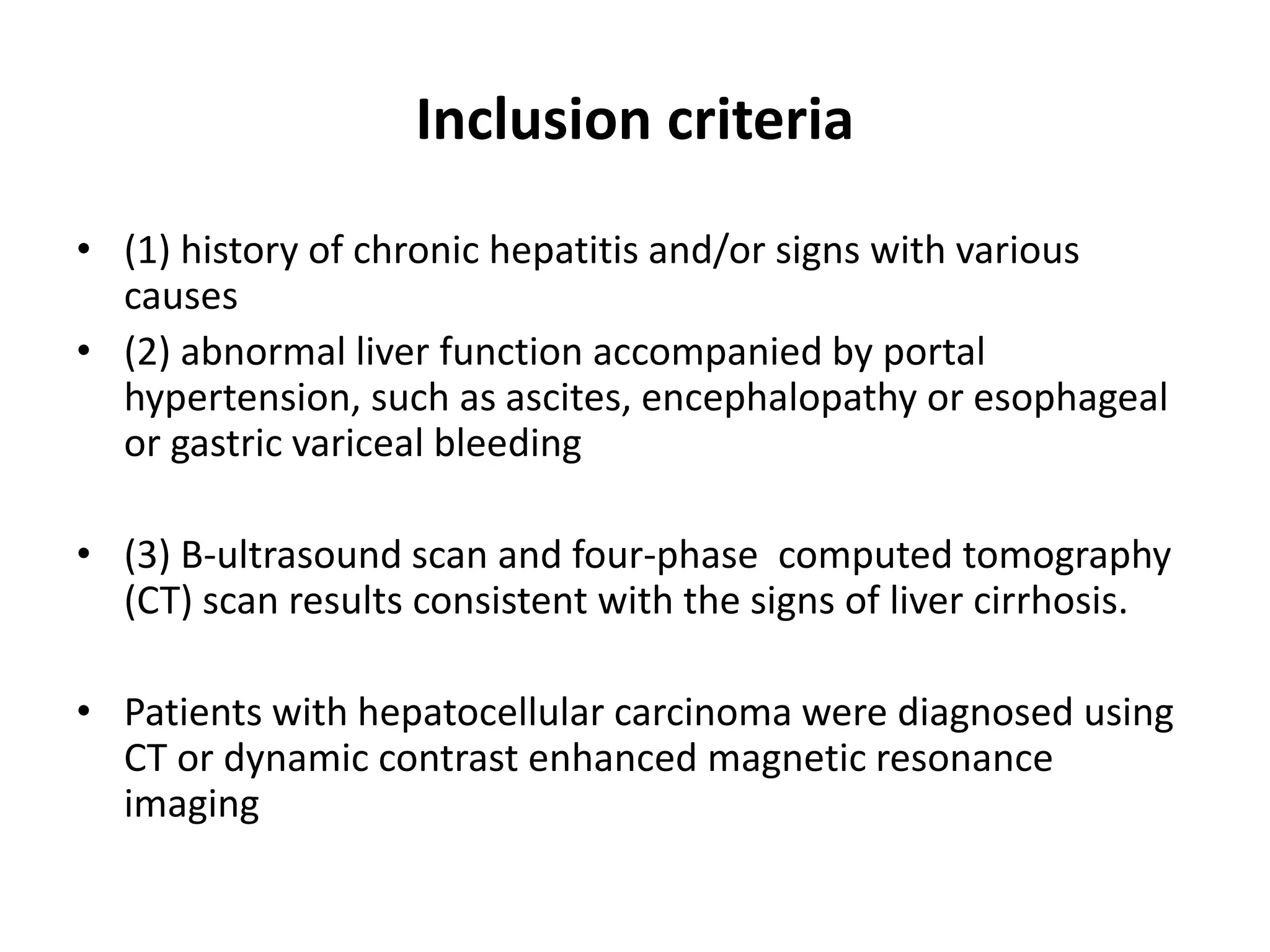
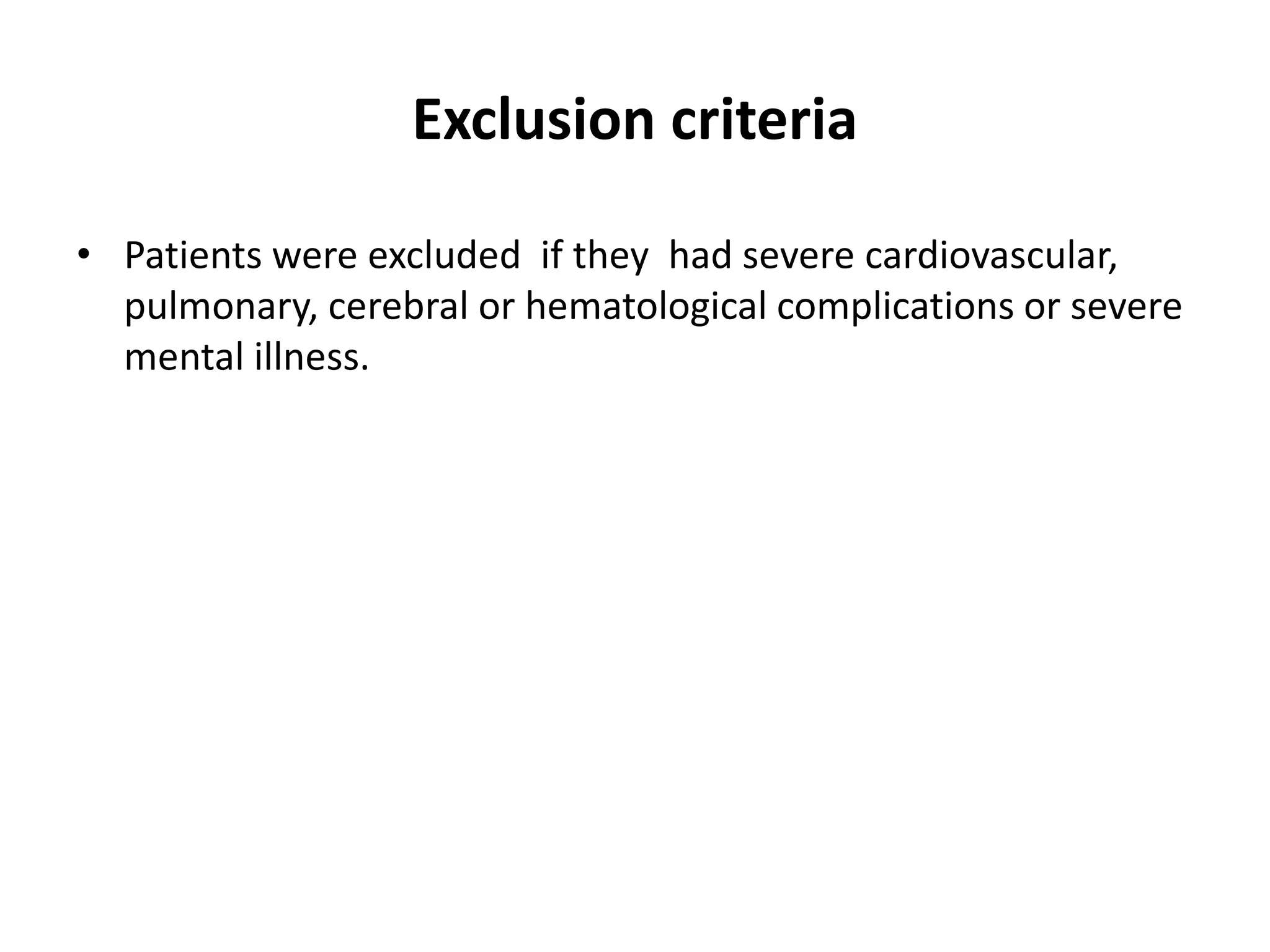
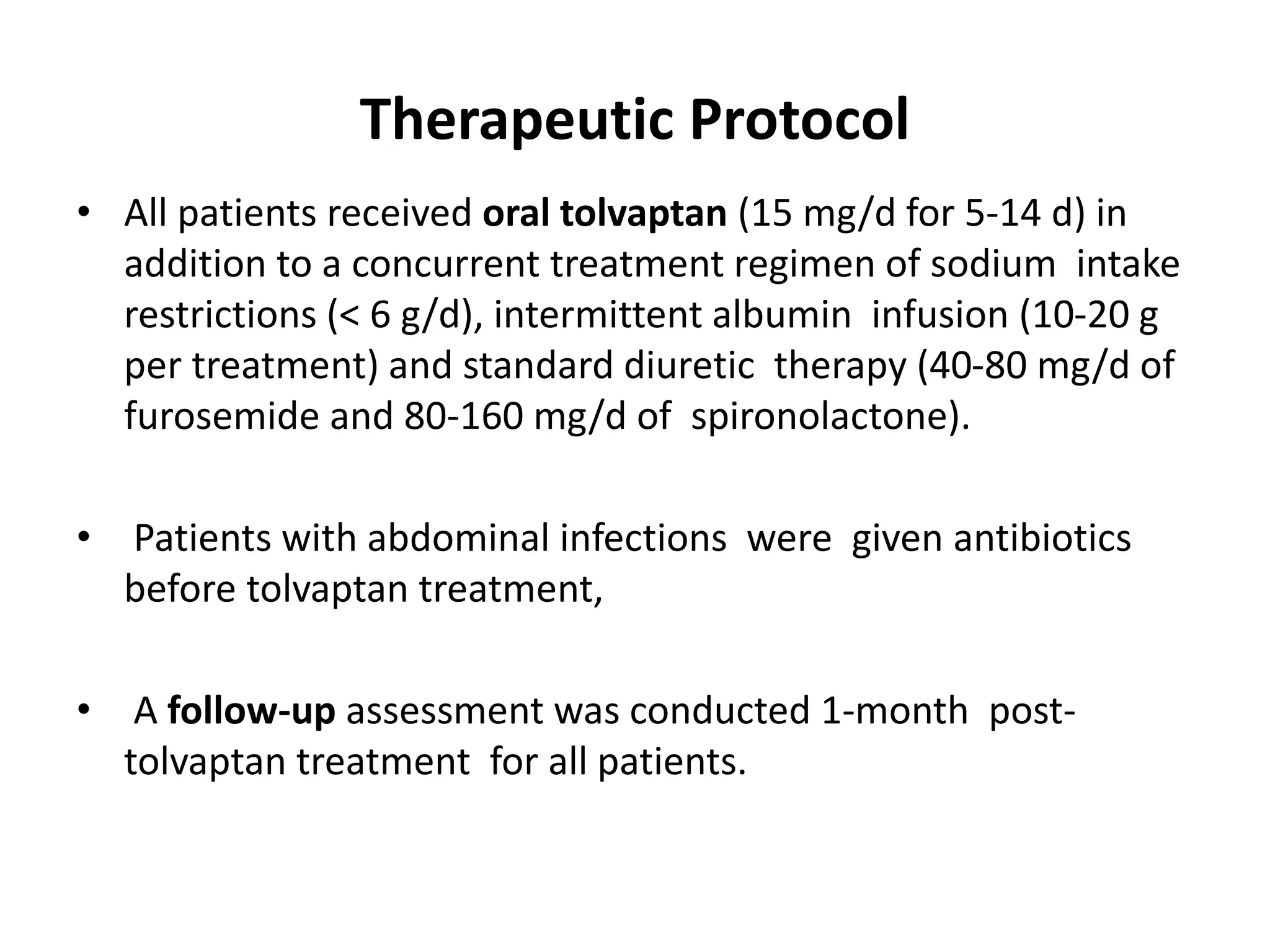
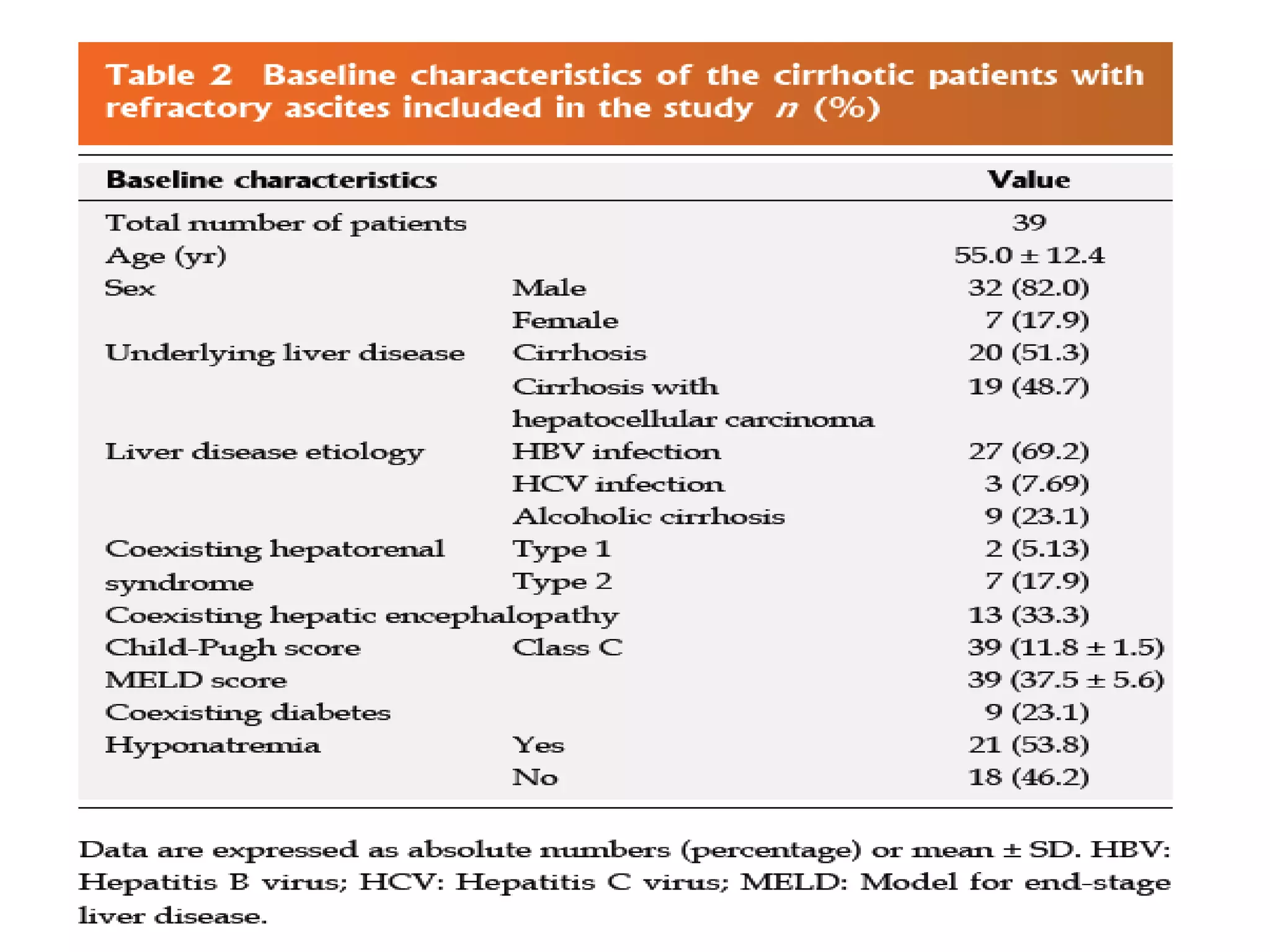
- Dr. Kazi Zakir Hossain presented on the clinical efficacy of tolvaptan for treating refractory ascites in liver cirrhosis patients.
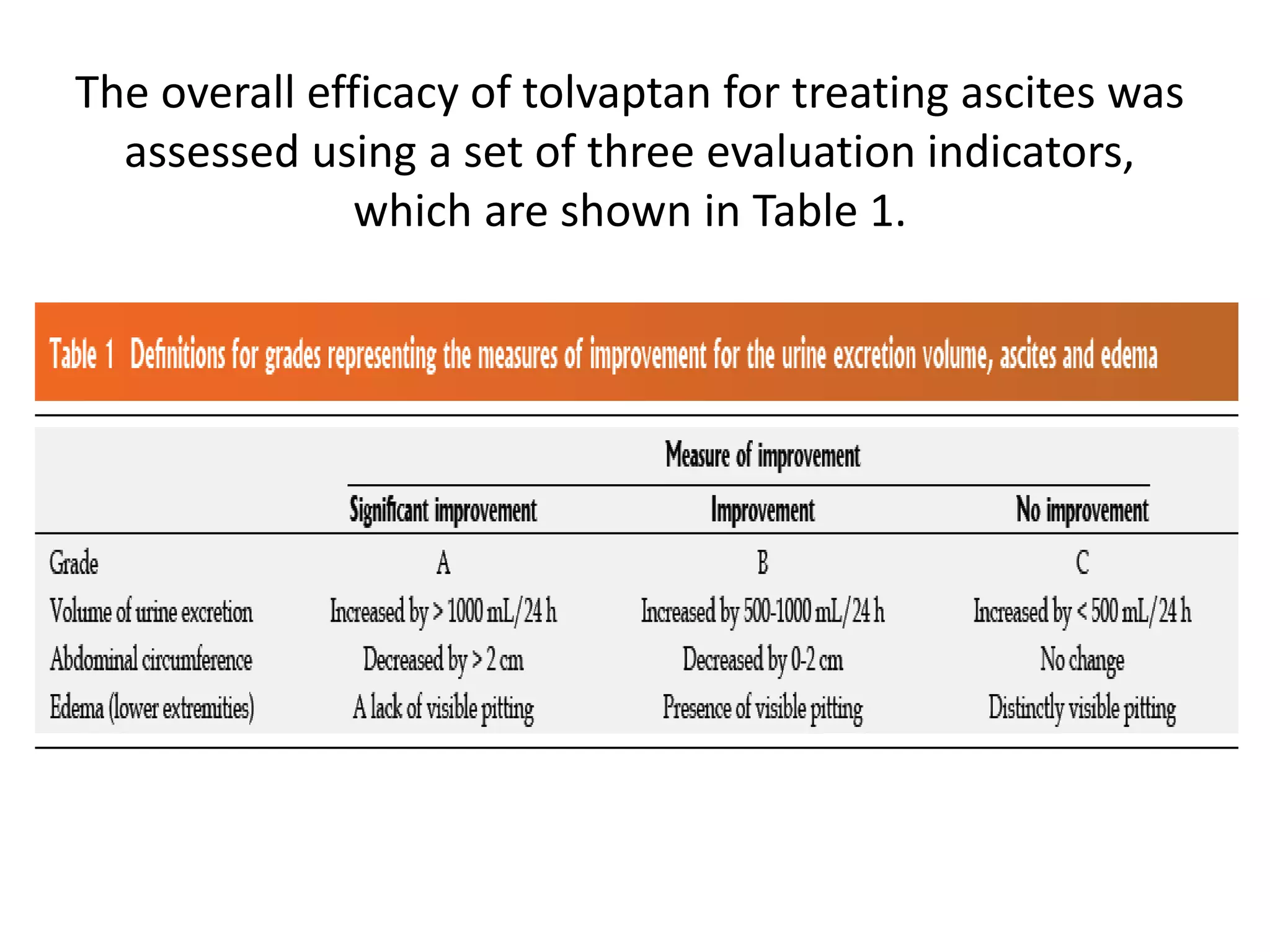
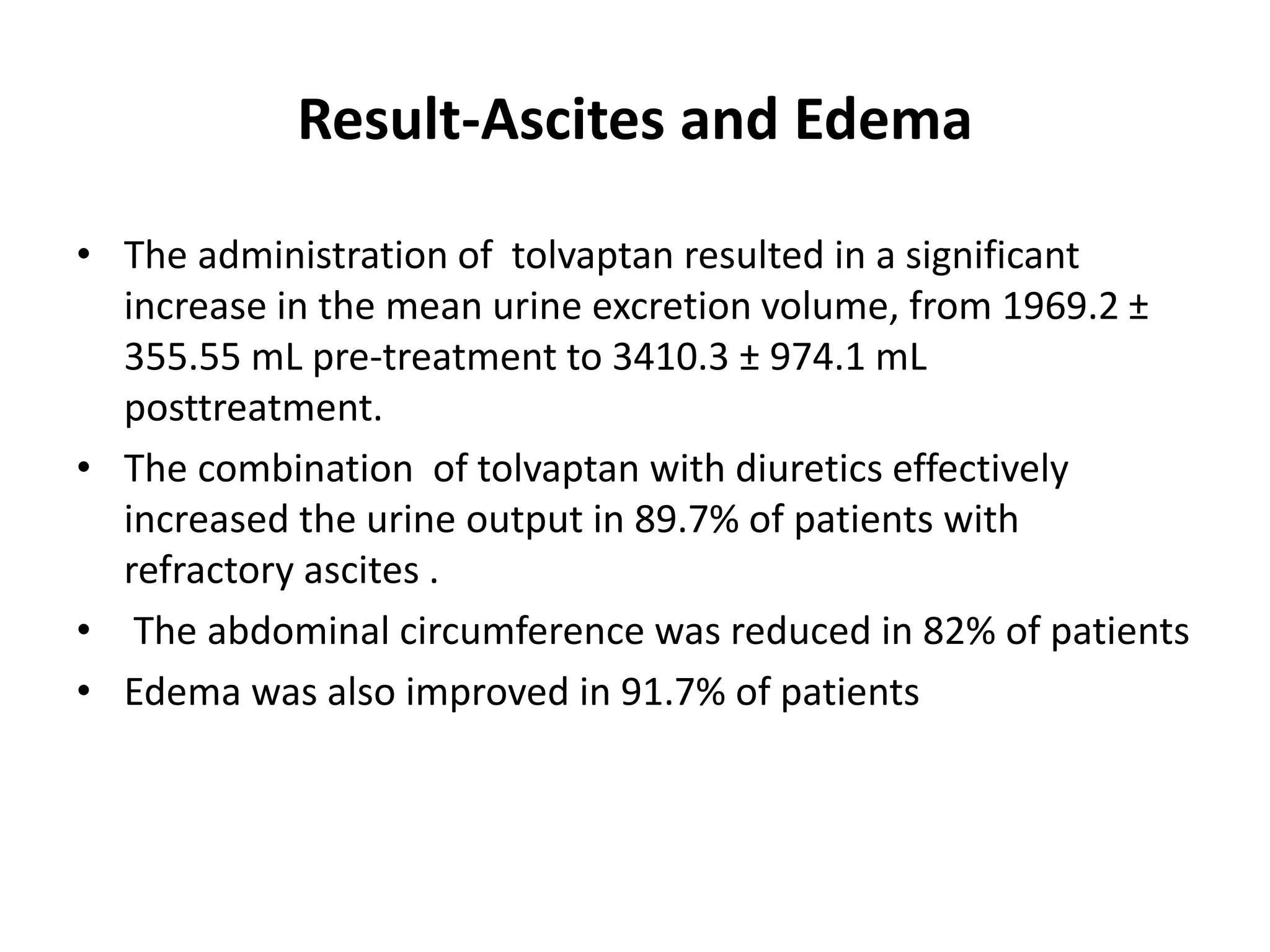
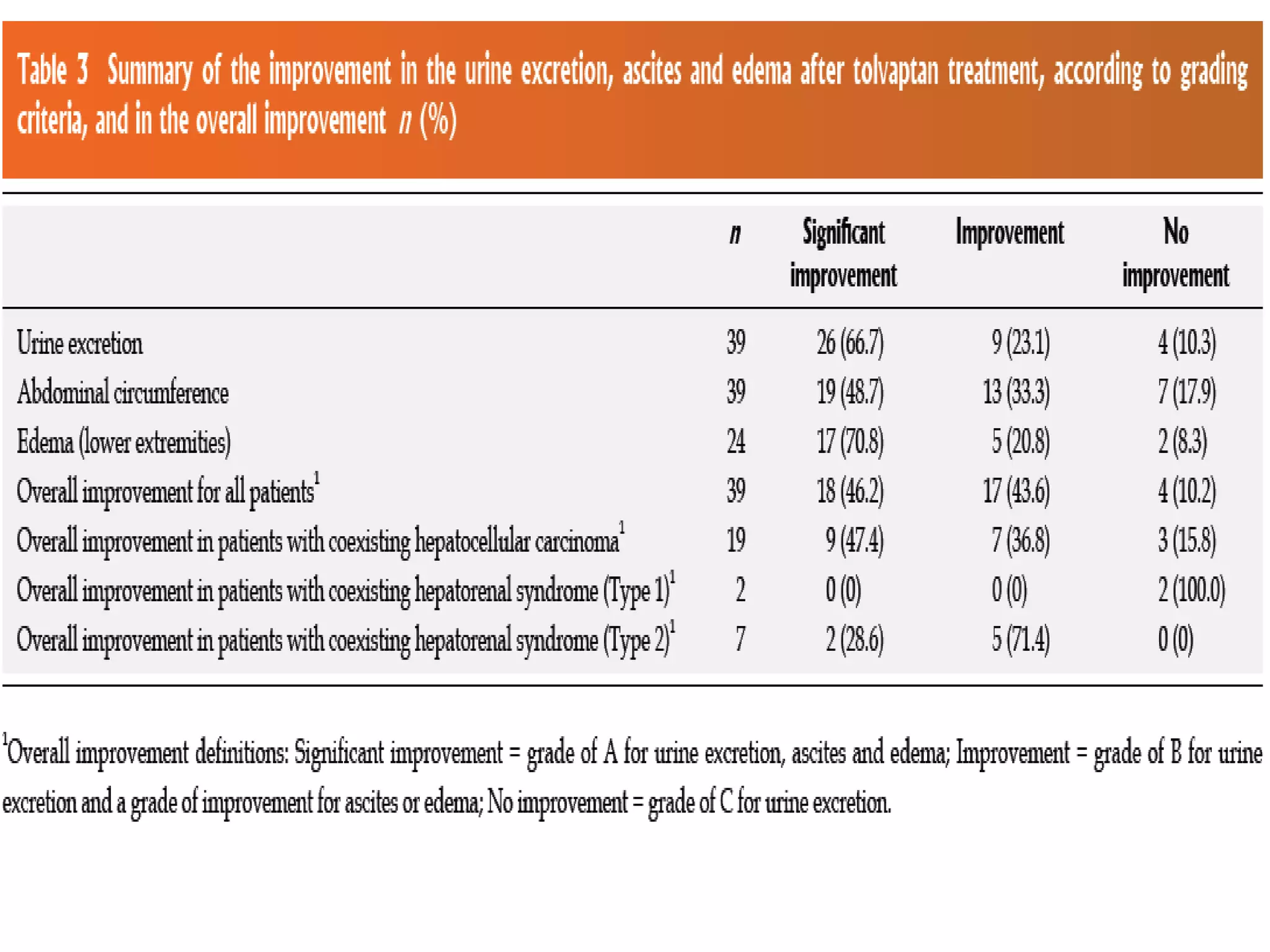
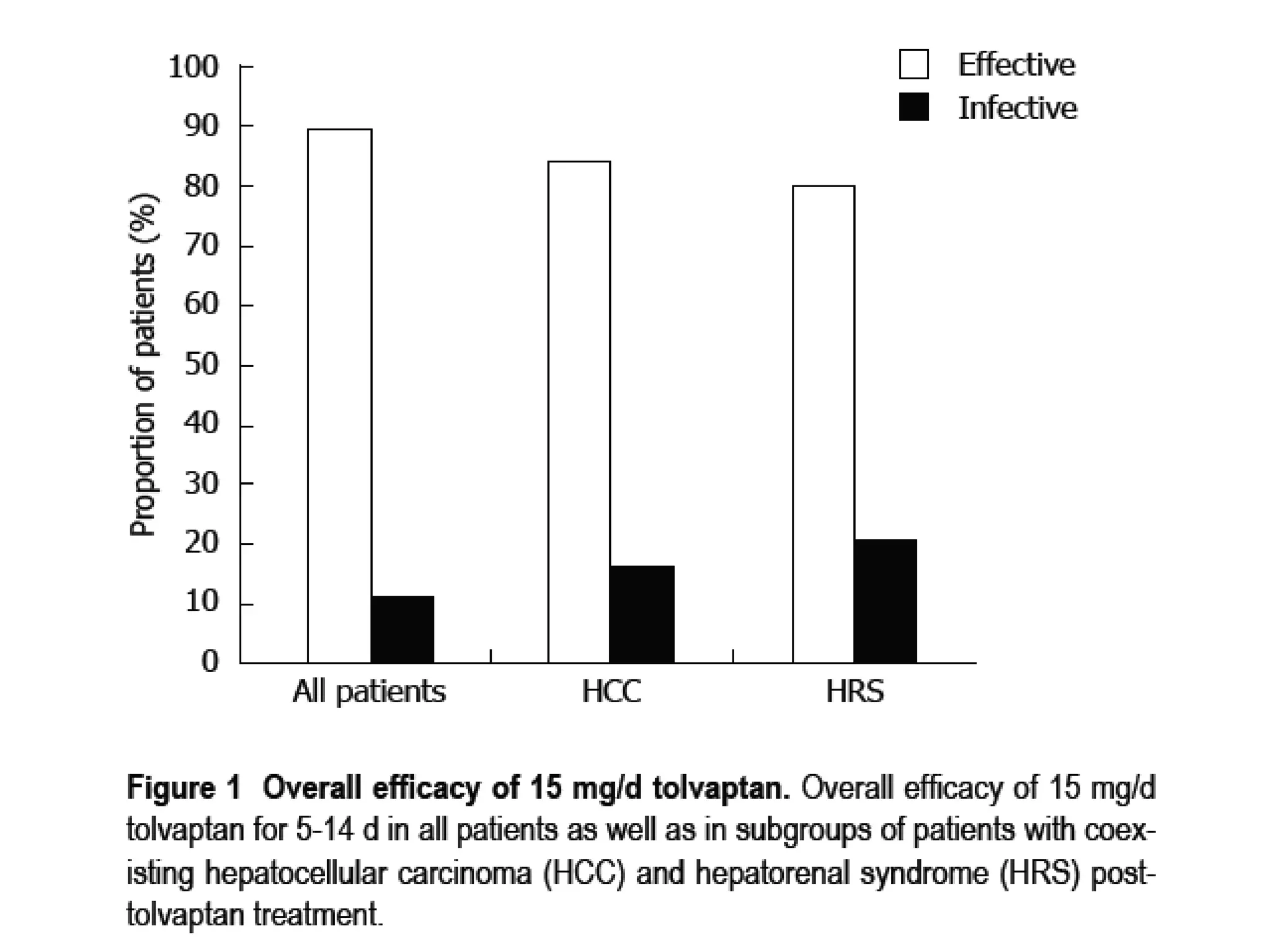
- The study found that tolvaptan combined with diuretics and sodium restrictions significantly increased urine output and reduced abdominal circumference and leg edema in patients with refractory ascites. It was effective in treating ascites in 89.7% of patients.
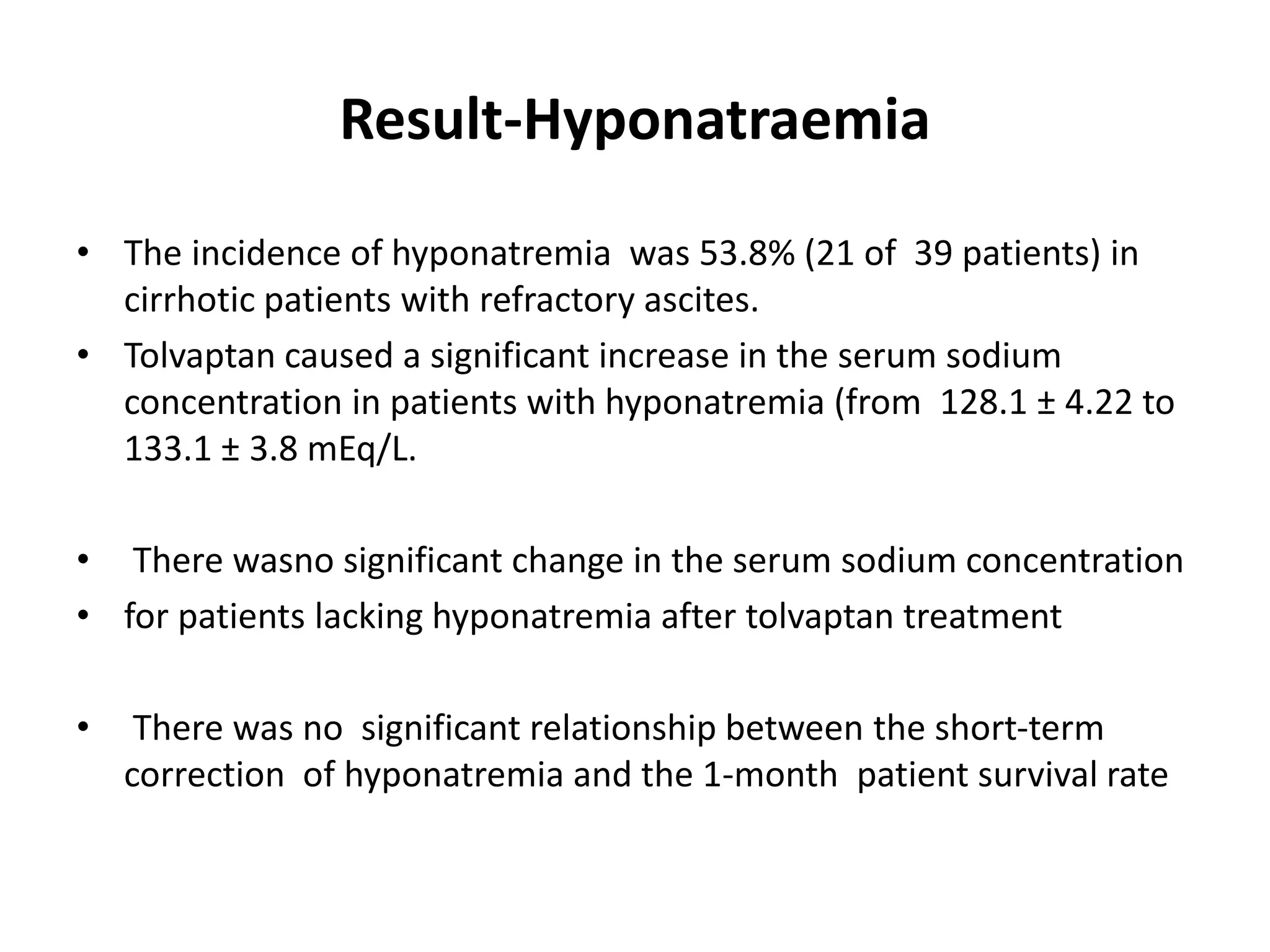
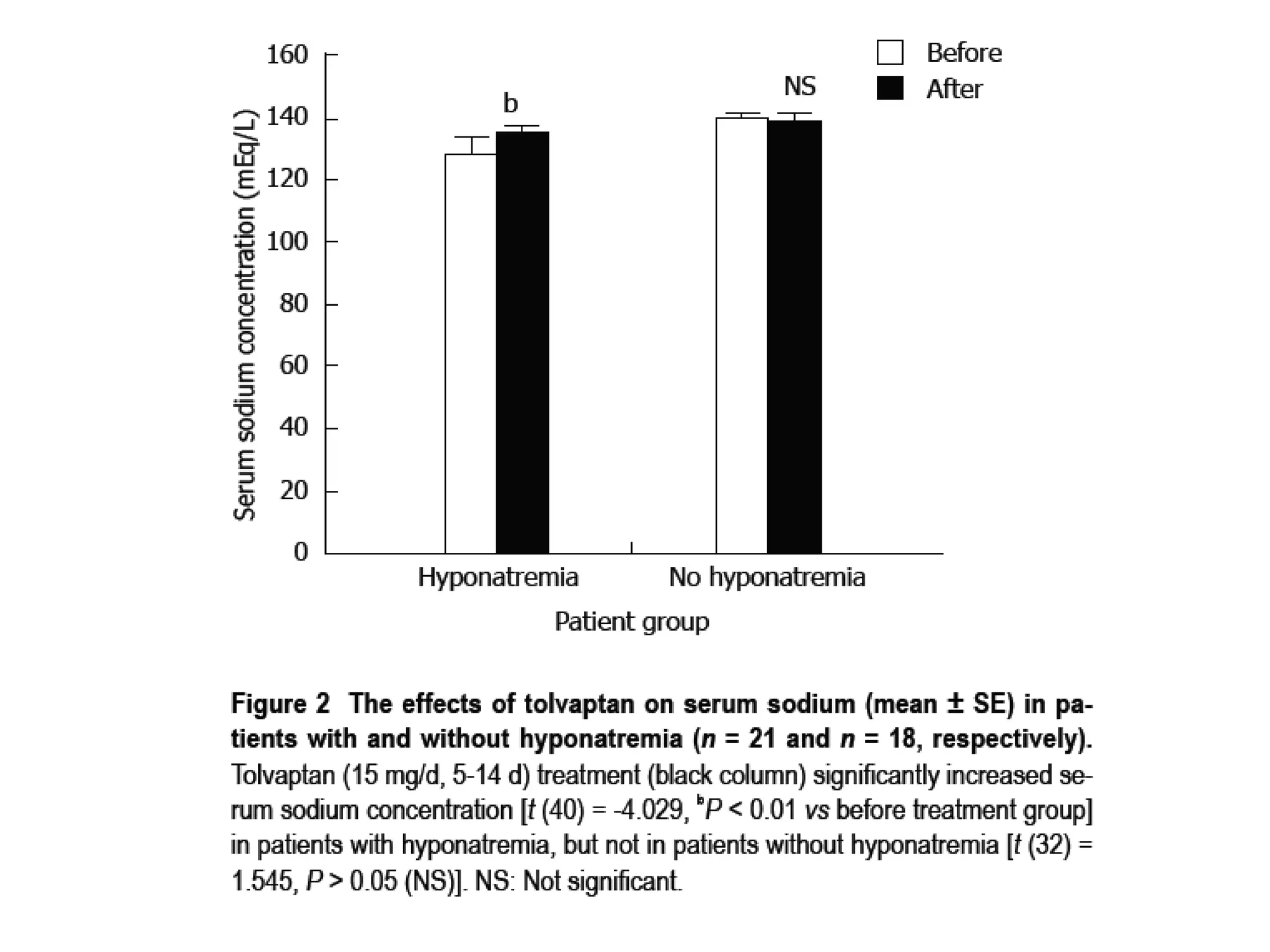
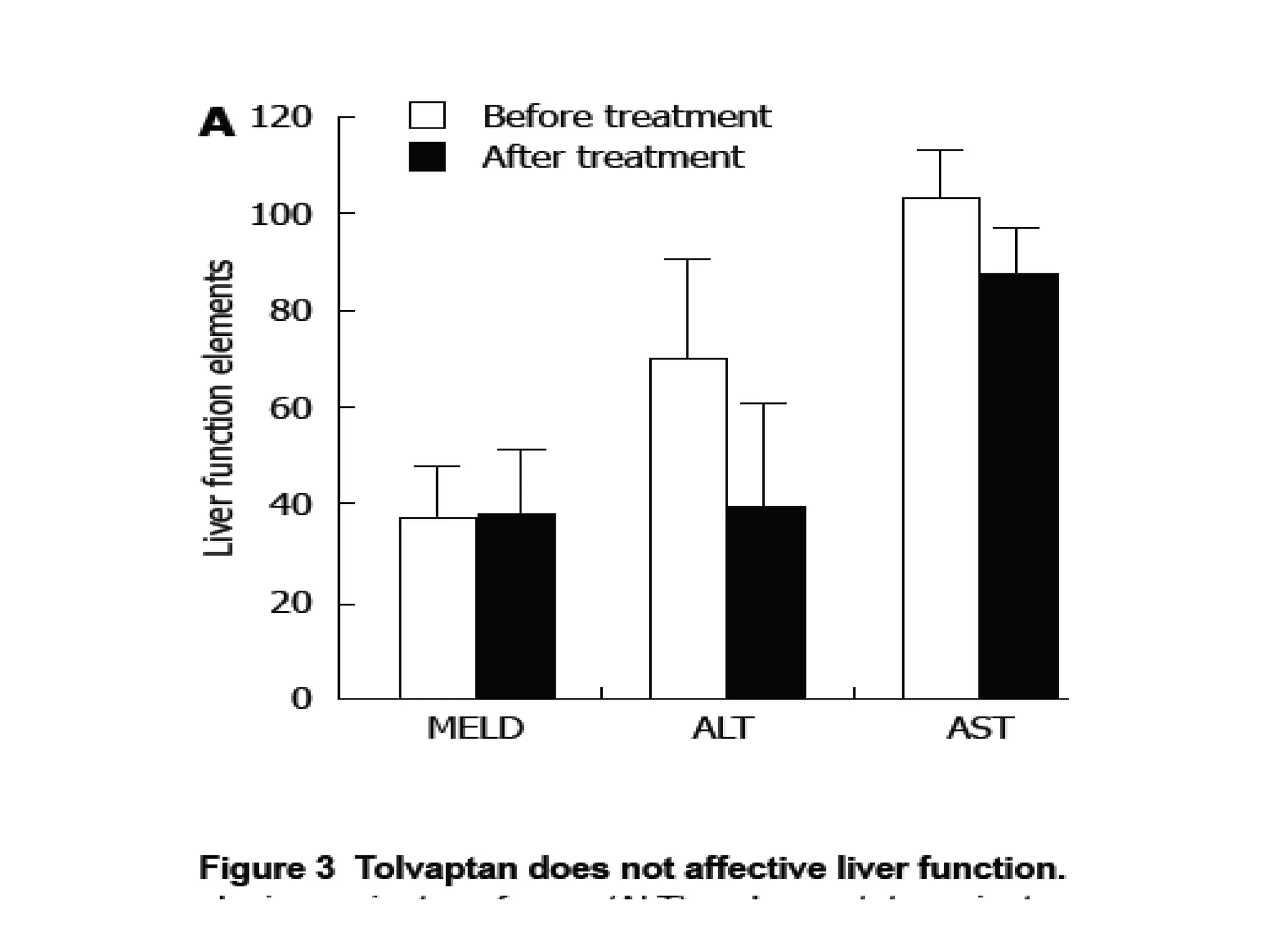
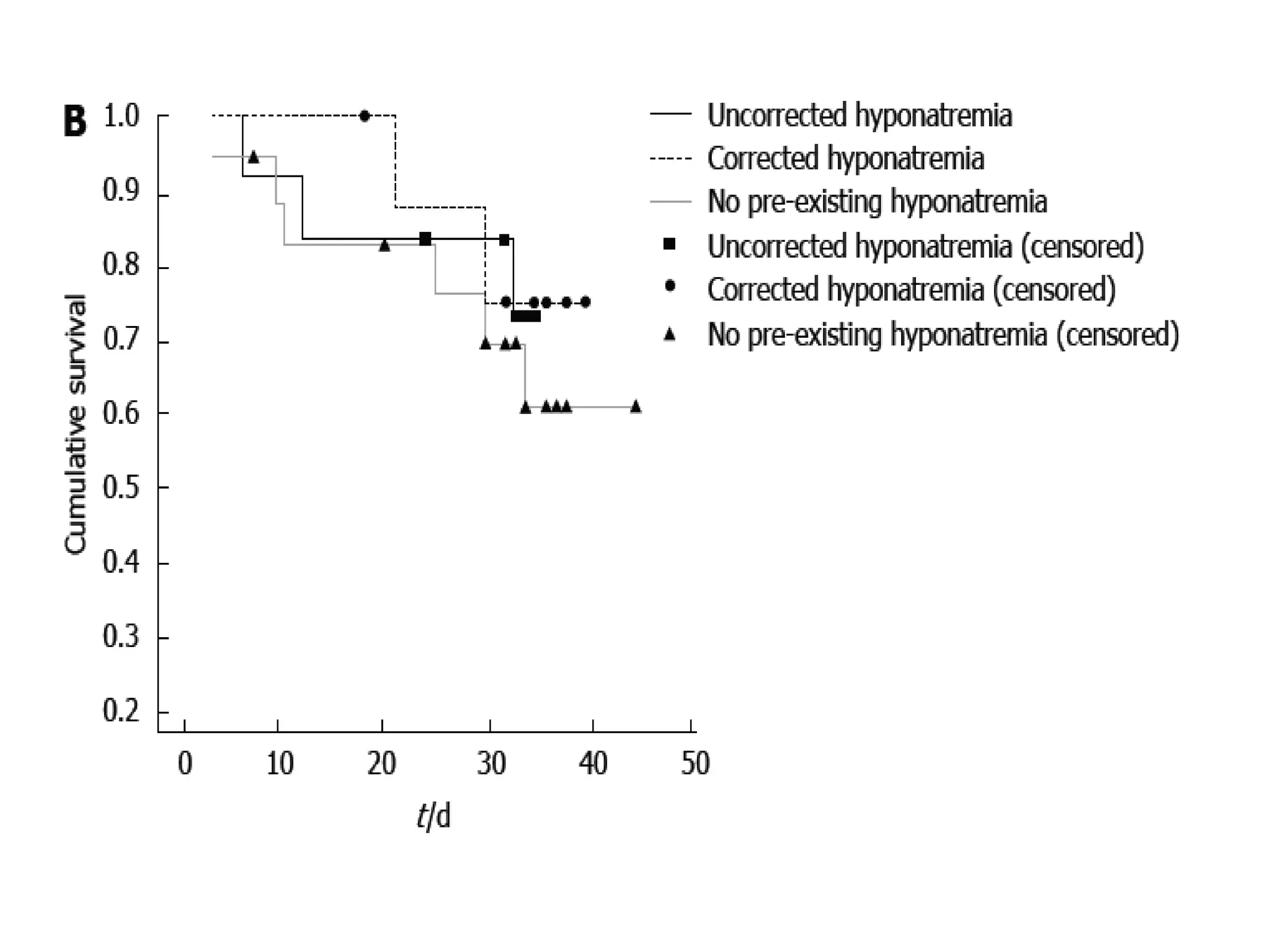
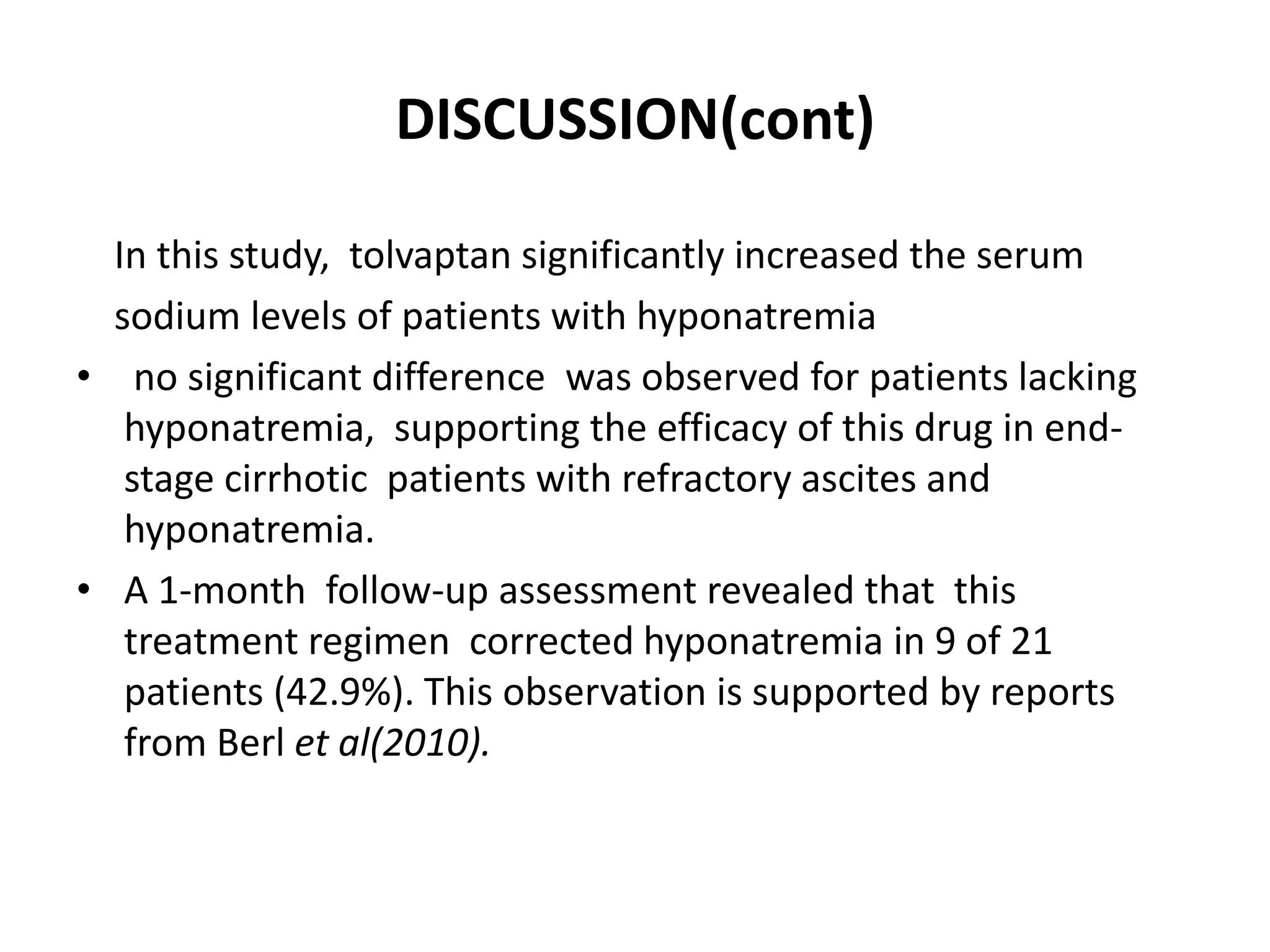
- Tolvaptan also significantly increased serum sodium levels in patients with hyponatremia but did not affect sodium levels in patients without hyponatremia. The treatment was generally well-tolerated with only mild thirst and dry mouth reported in a few patients.