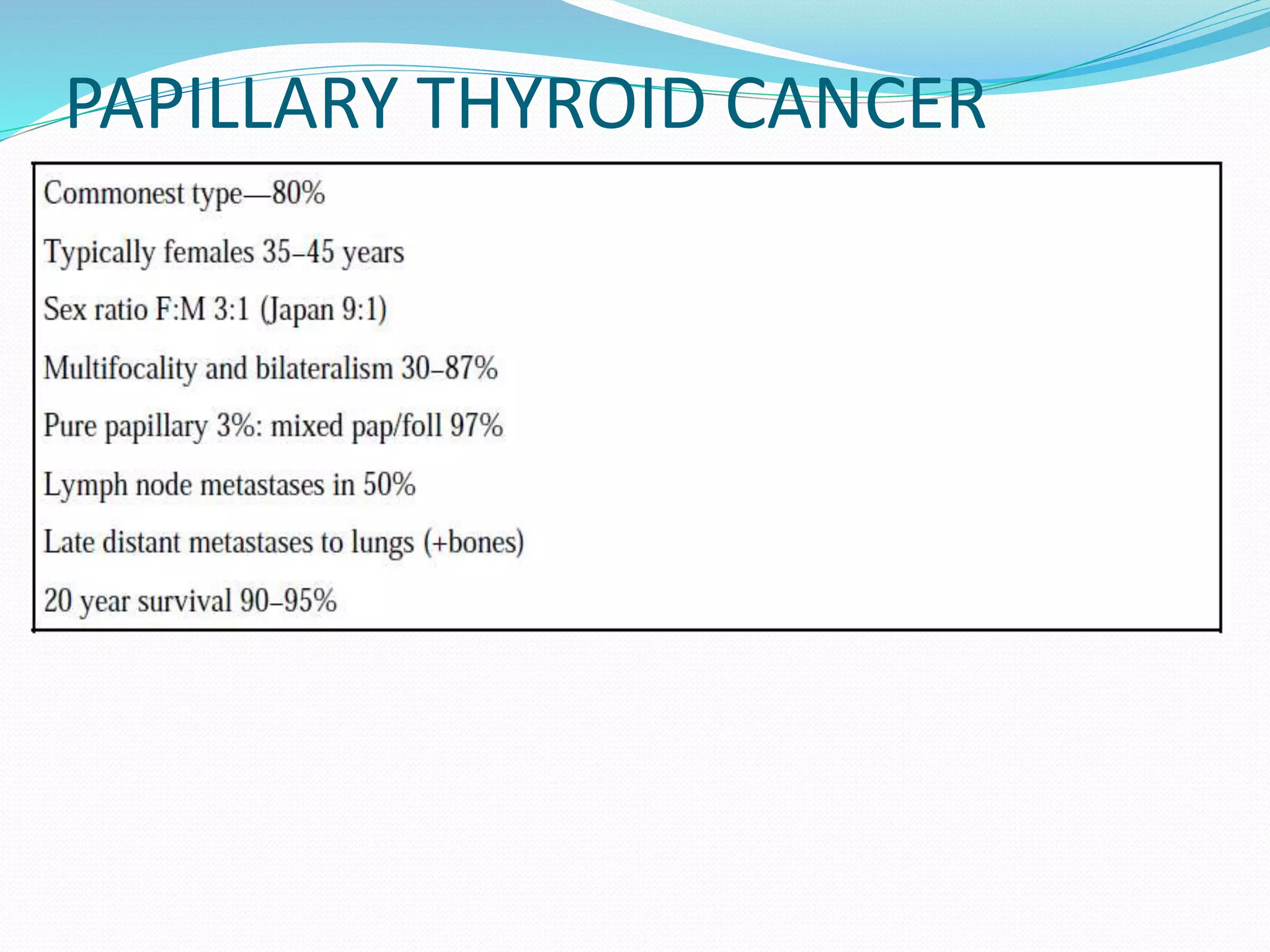
The document discusses the anatomy, blood supply, lymph drainage, etiology, pathological classification, clinical presentation, investigative workup, staging systems, and management of thyroid cancer. It provides details on the location and structure of the thyroid gland. It describes the different types of thyroid cancers including papillary, follicular, hurthle cell, and anaplastic carcinoma. It discusses the role of surgery, radioactive iodine therapy, and neck dissection in the treatment of thyroid cancer.