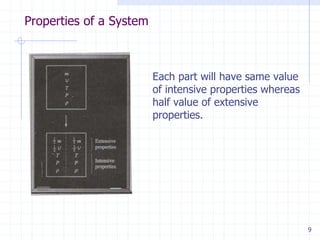
This document provides an overview of key concepts in thermodynamics taught by Professor Yash B. Parikh. It defines homogeneous and heterogeneous systems, pure substances, dimensions and units (including the SI system), various types of energy (internal, external, chemical, atomic, molecular), intensive and extensive properties, specific properties, states of a system, equilibrium, processes and cycles (including quasi-static processes and iso- processes where a property remains constant like isothermal, isobaric, isometric).