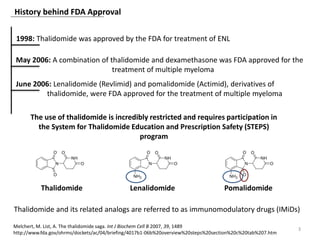
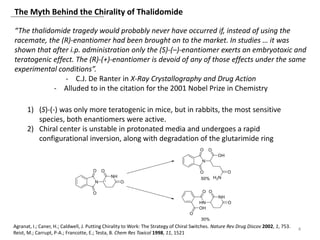
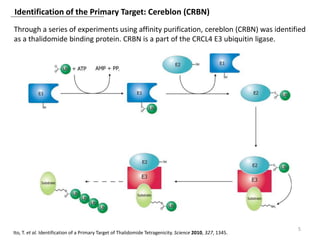
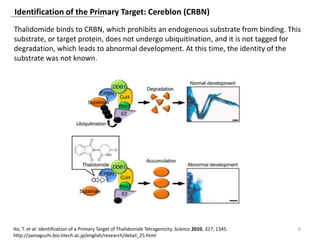
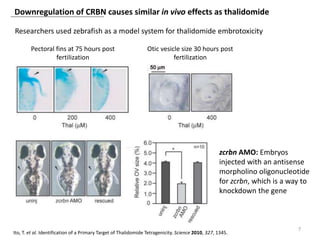
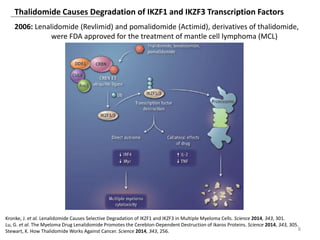
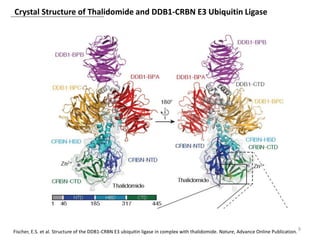
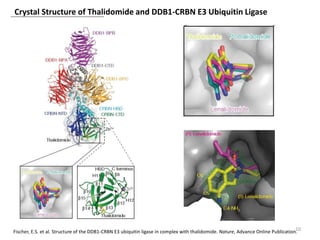
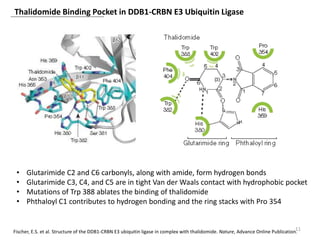
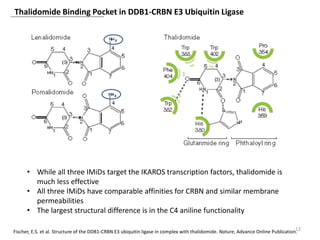
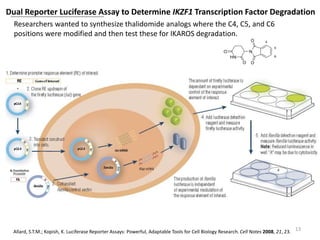
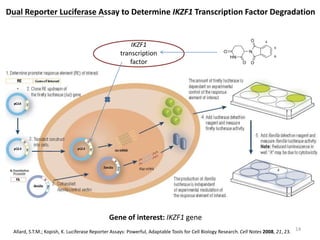
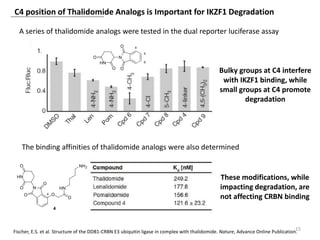
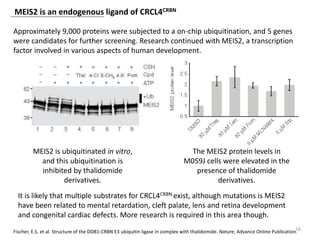
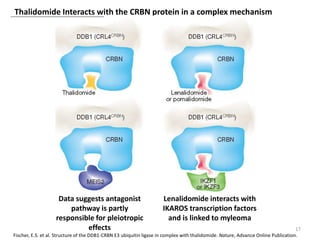
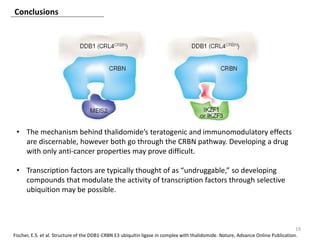
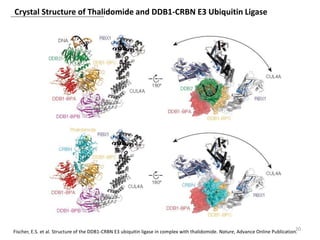
The document outlines the history of thalidomide, detailing its initial approval for use, subsequent ban due to birth defects, and later FDA approvals for cancer treatments. It highlights the identification of cereblon (CRBN) as the primary target of thalidomide's effects, including its teratogenic and anti-cancer properties. Additionally, it discusses the complex interactions and mechanisms involved in thalidomide's action, suggesting challenges in developing drugs that separate its therapeutic and toxic effects.