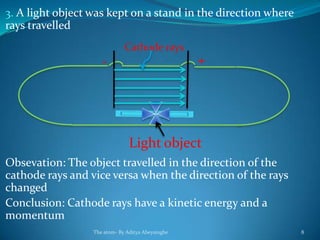
1) Scientists like Faraday, Crookes, and Plucker studied the behavior of atoms and molecules using electric currents passed through liquids and gases.
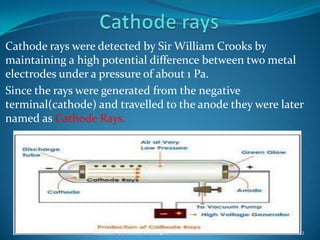
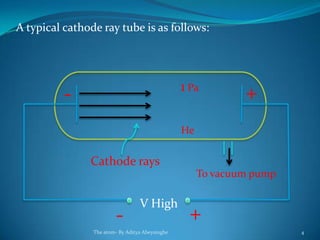
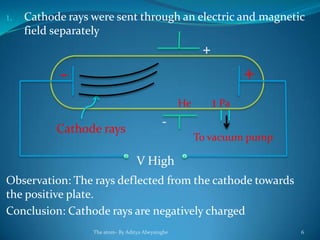
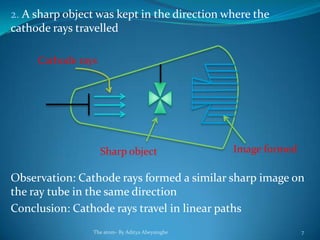
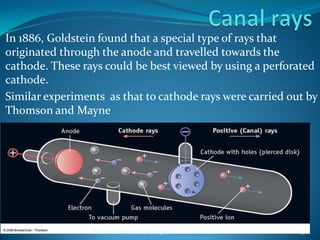
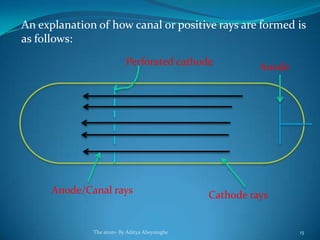
2) Cathode rays were discovered by Crookes within evacuated tubes, where a stream of electrons emitted from the negative cathode terminal. J.J. Thomson later discovered that cathode rays consisted of negatively charged particles called electrons through experiments showing they were deflected by electric and magnetic fields.
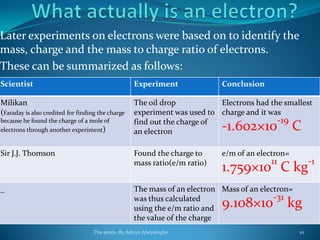
3) Additional experiments determined that electrons have negligible mass but a charge of -1.6×10^-19 coulombs, identifying them as fundamental building blocks of atoms and matter.