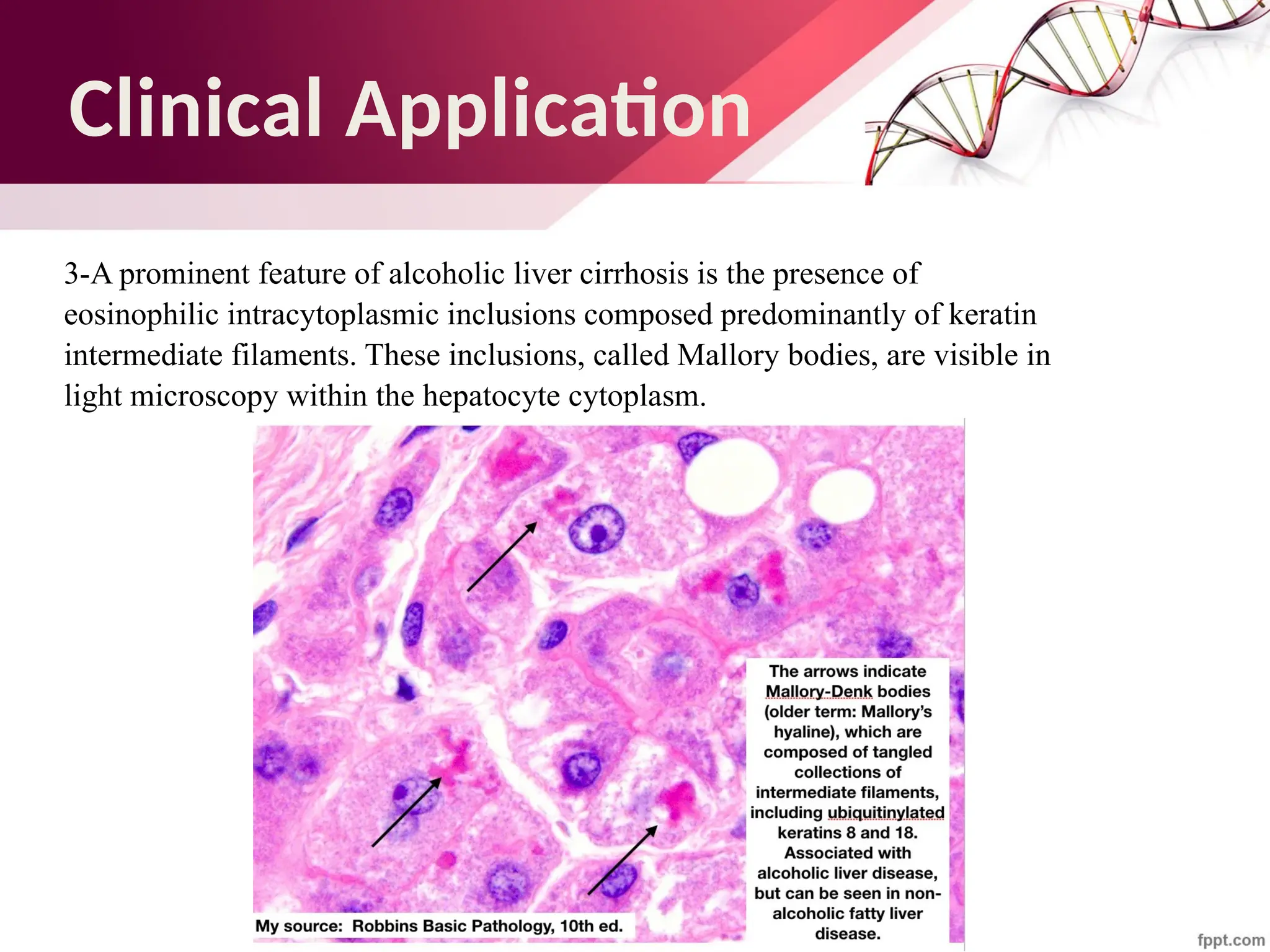
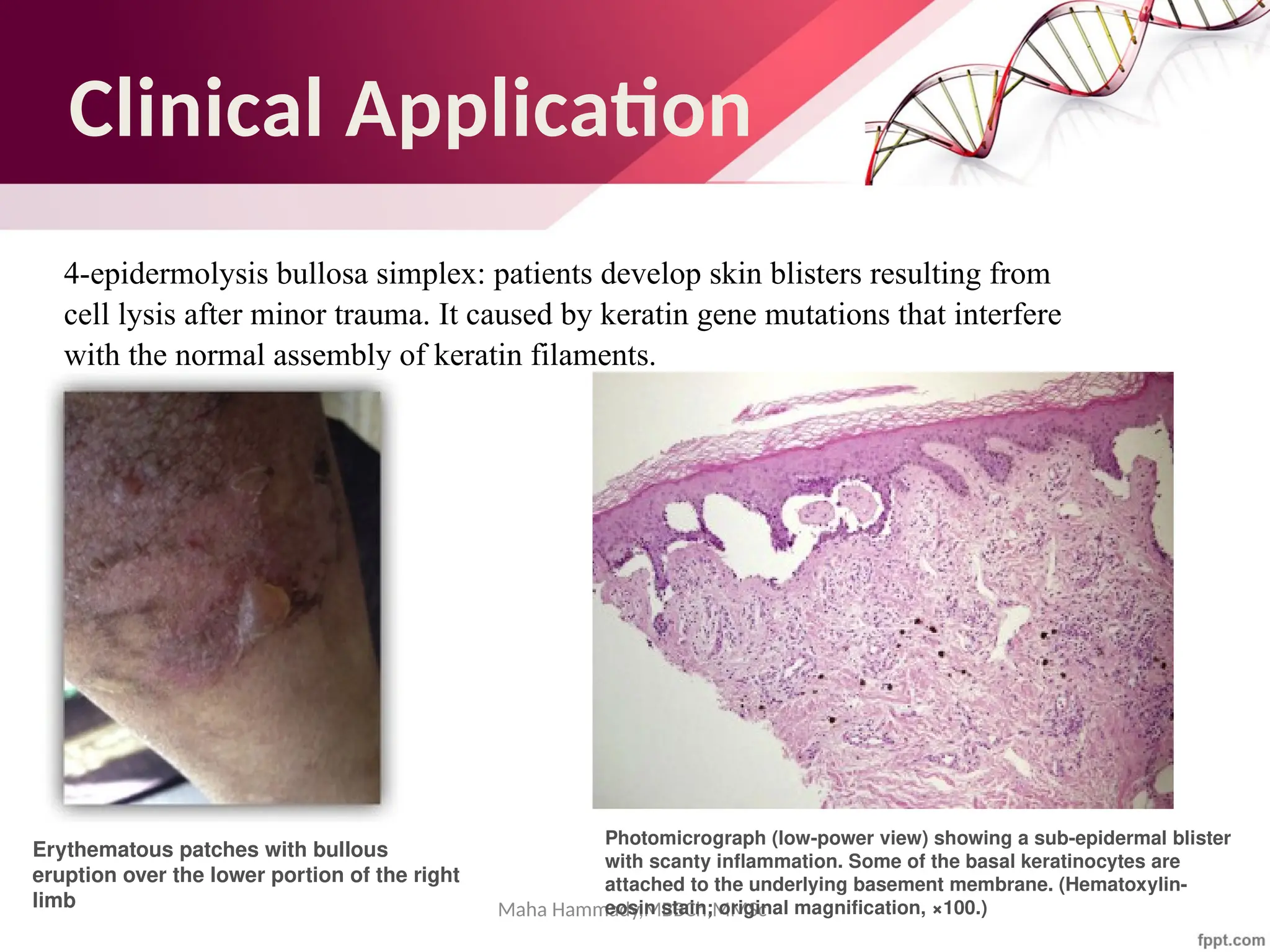
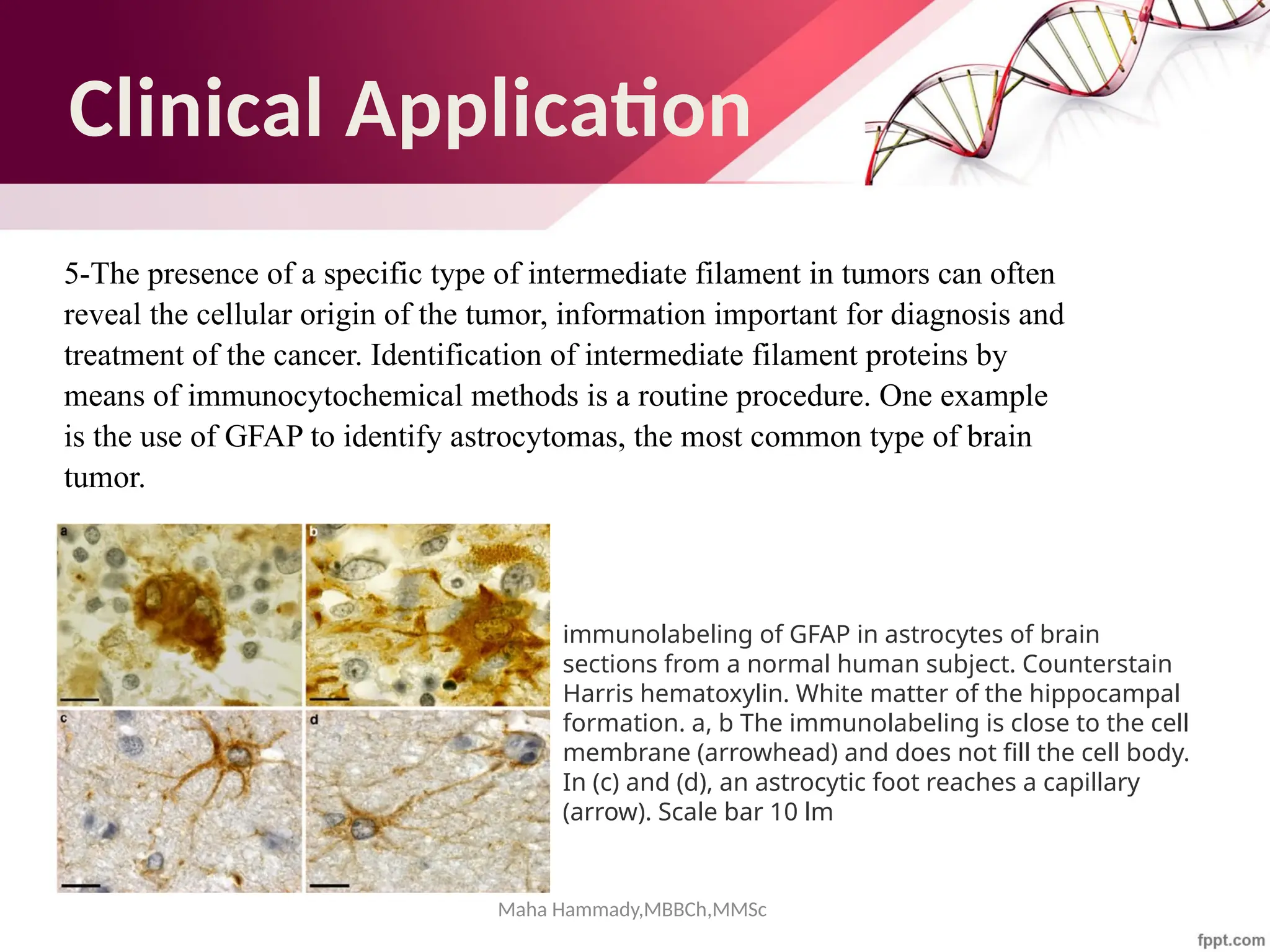
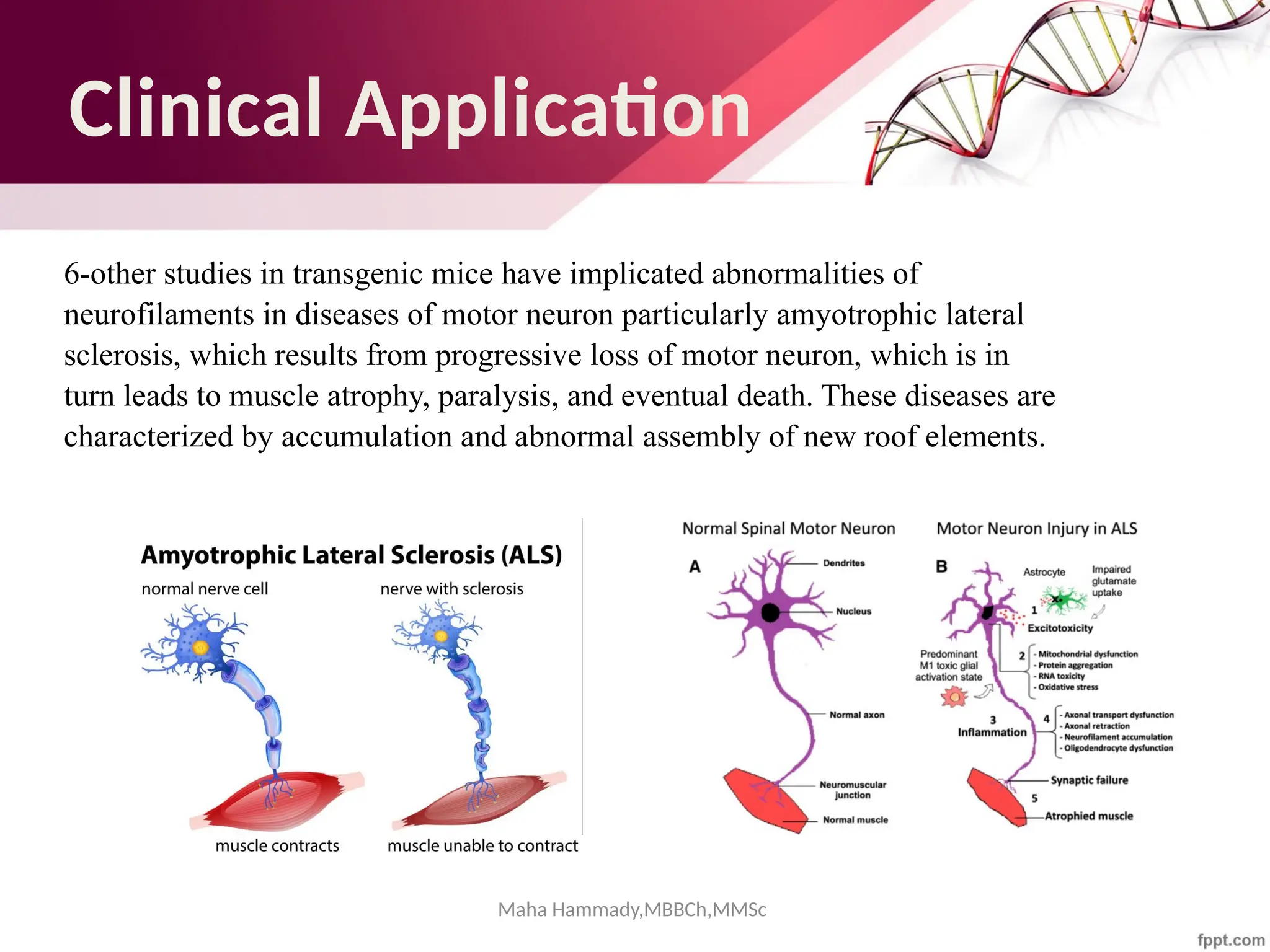
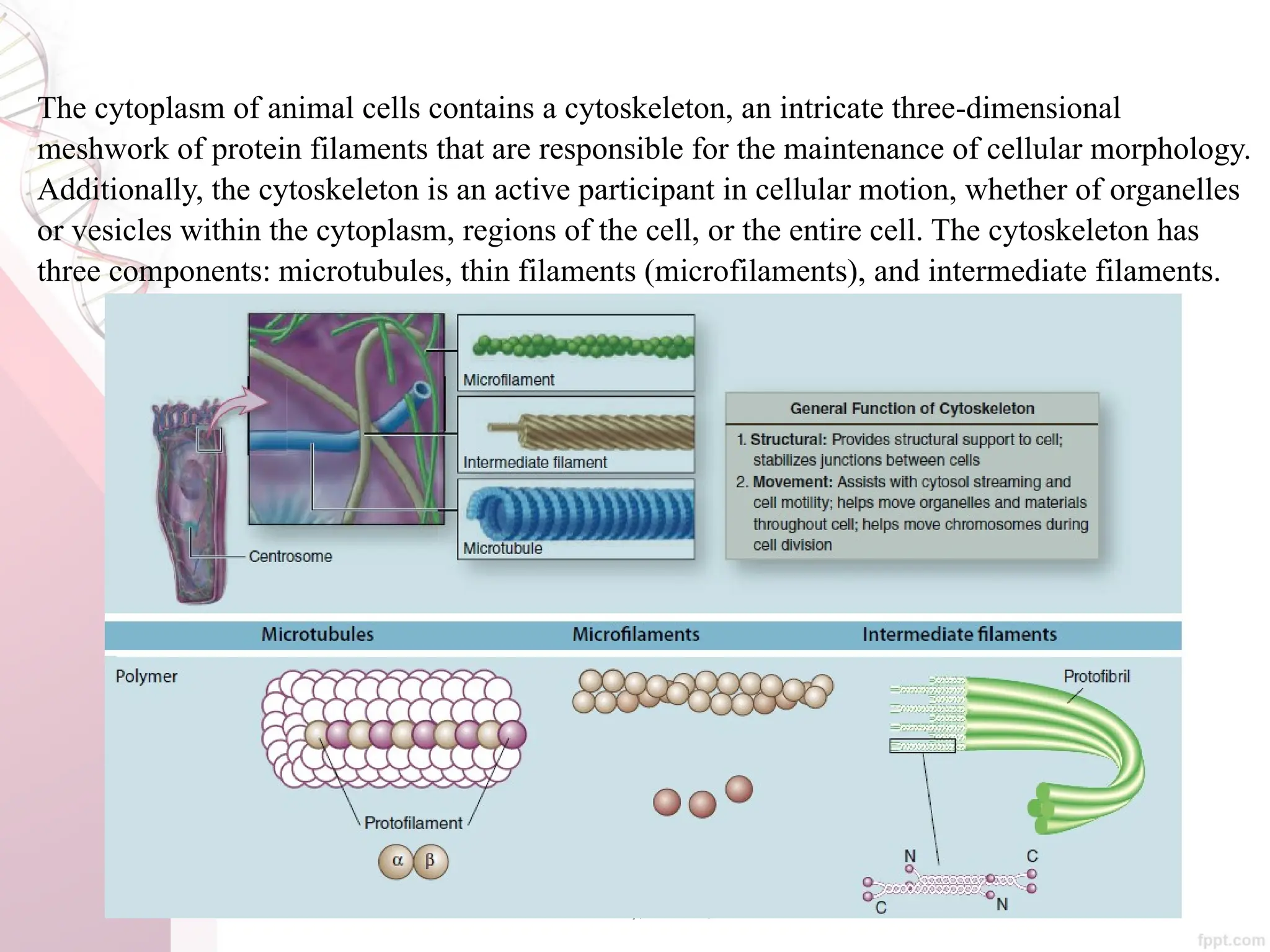
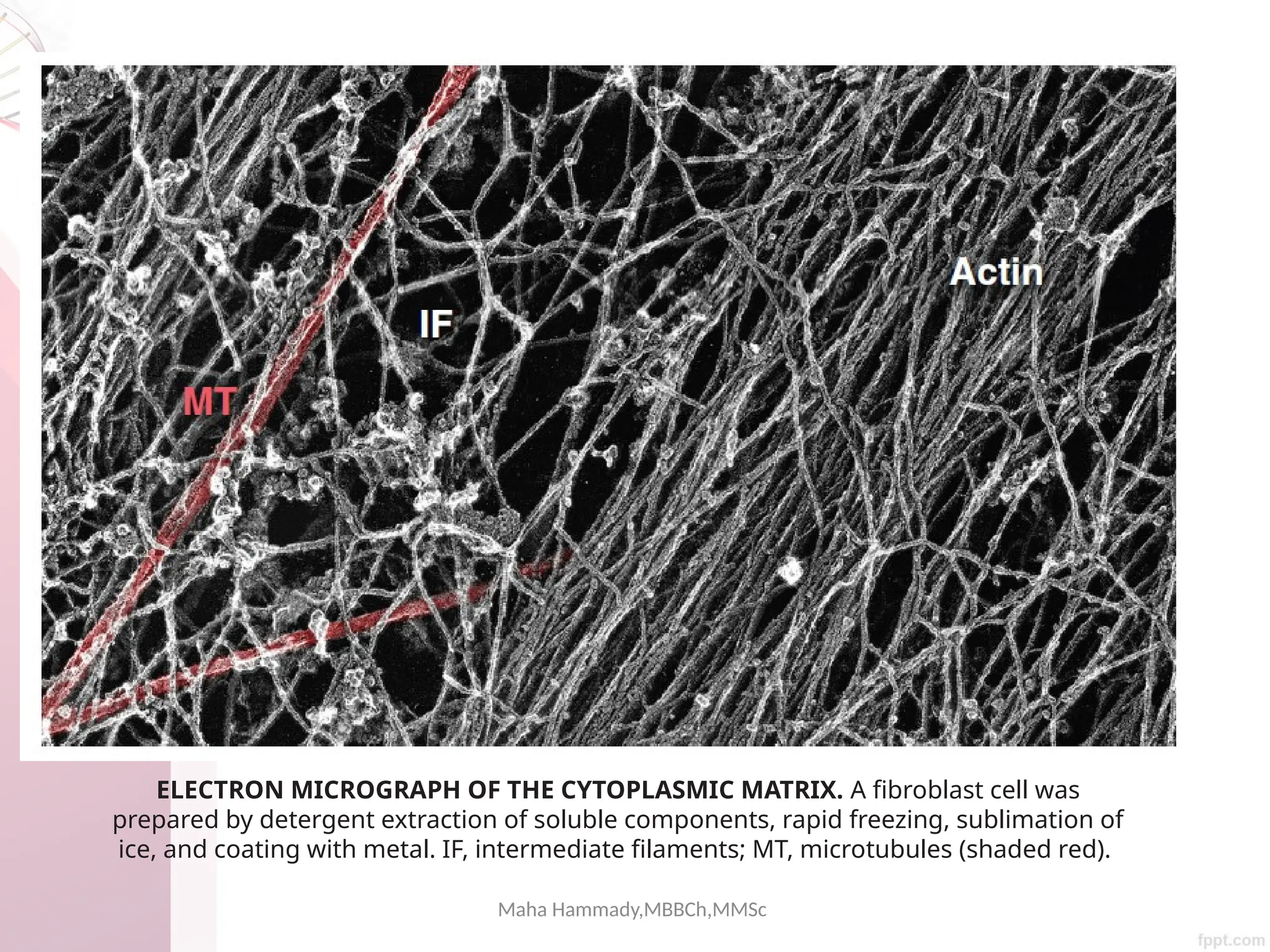
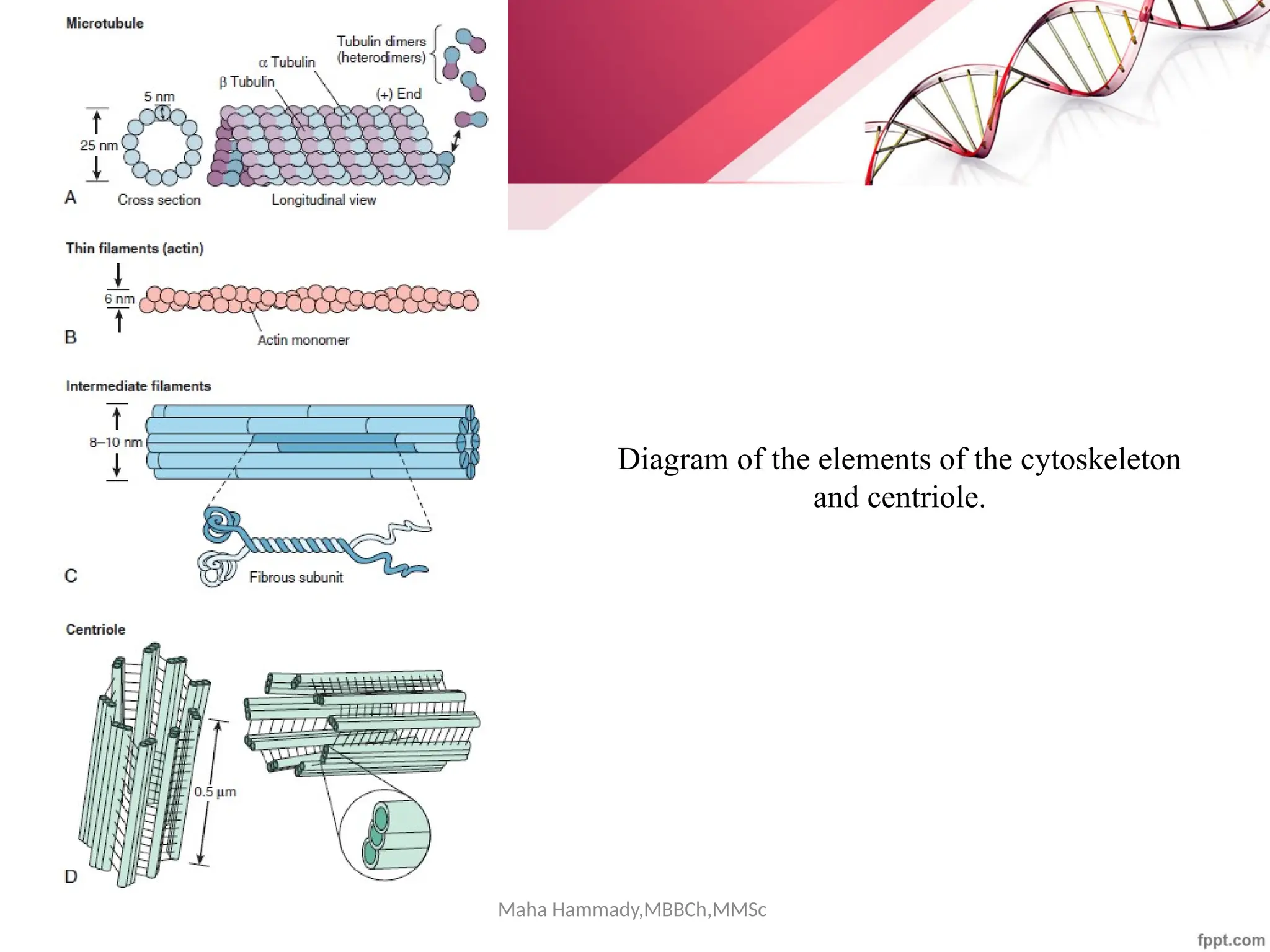
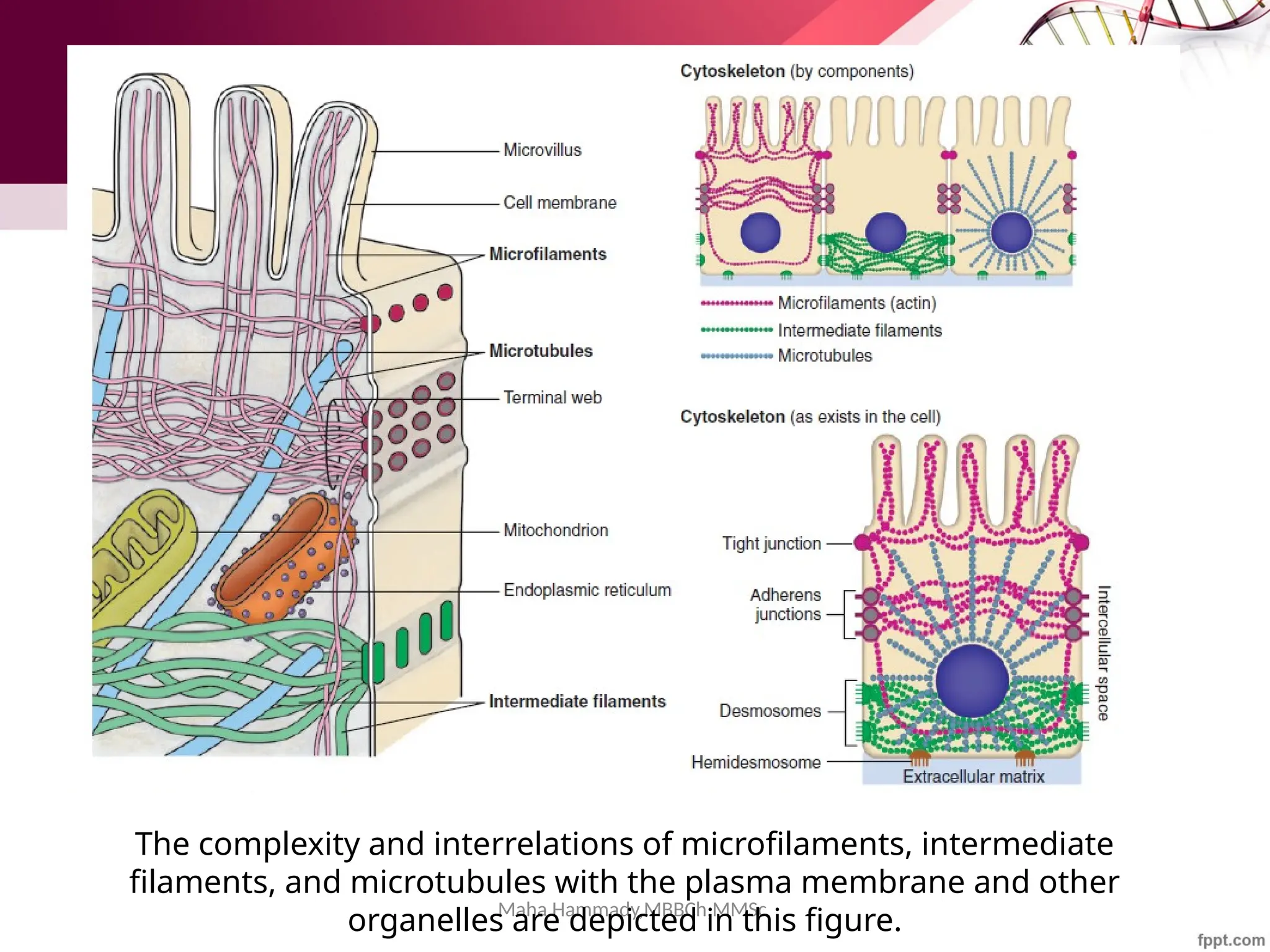
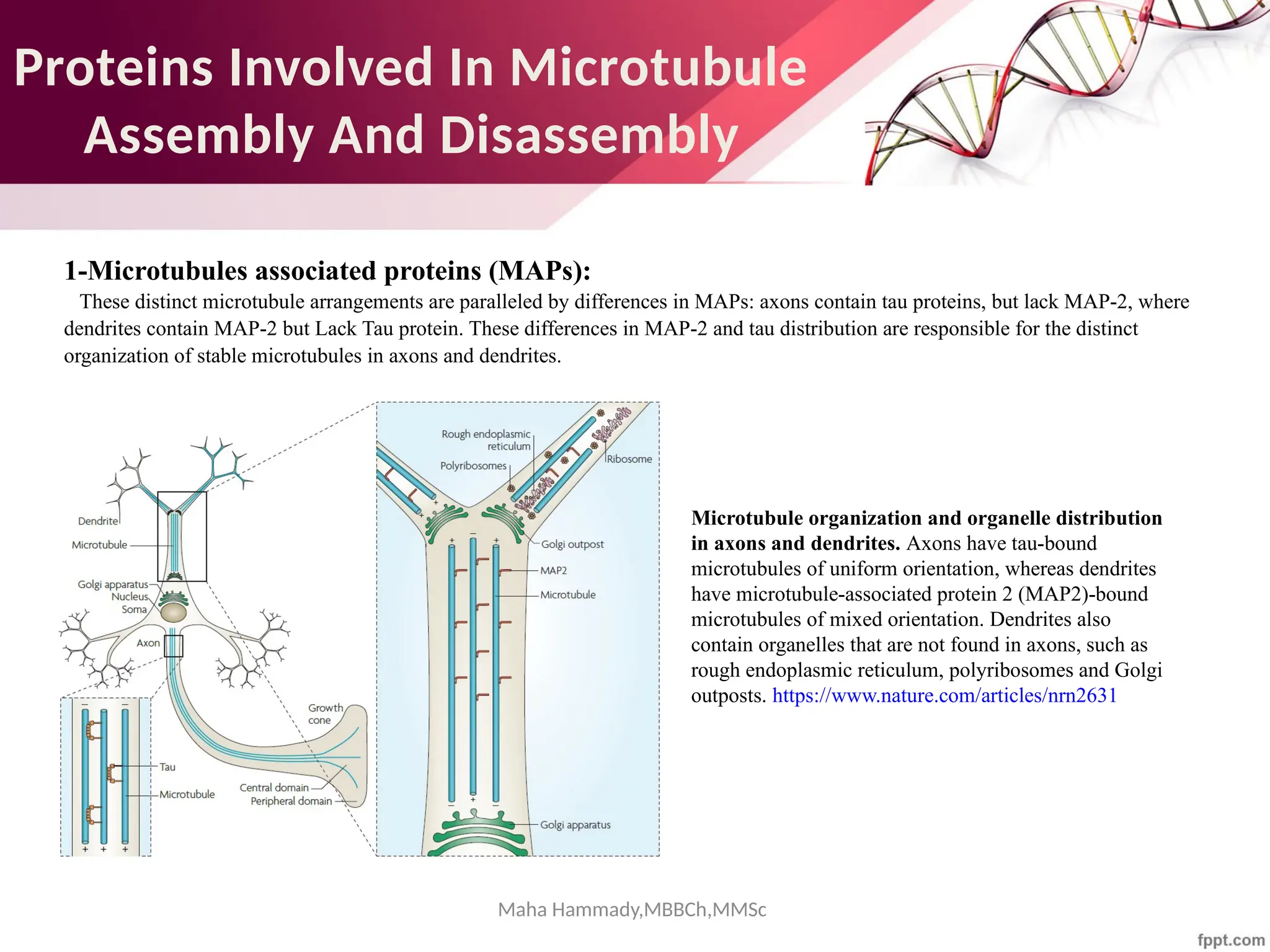
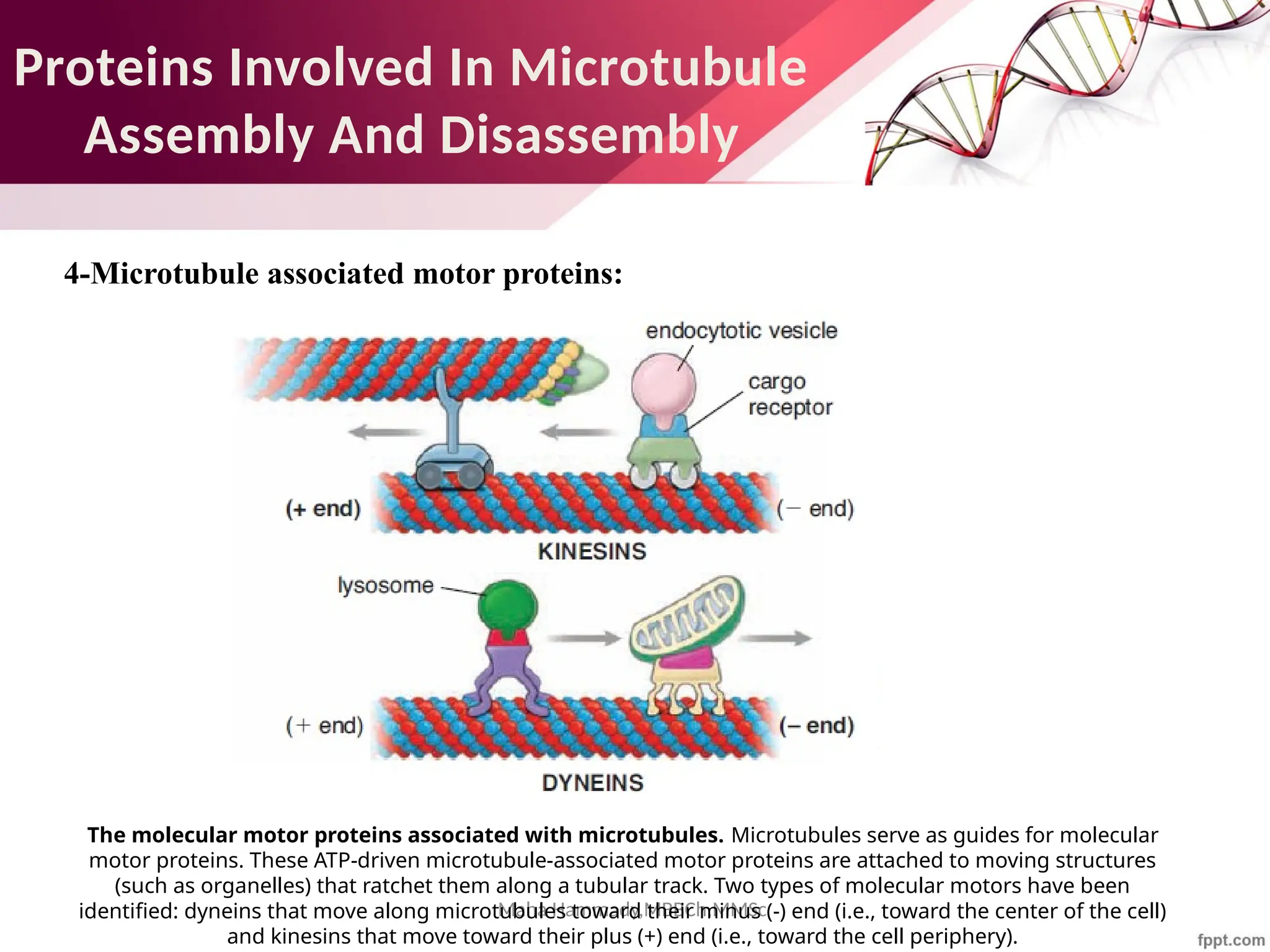
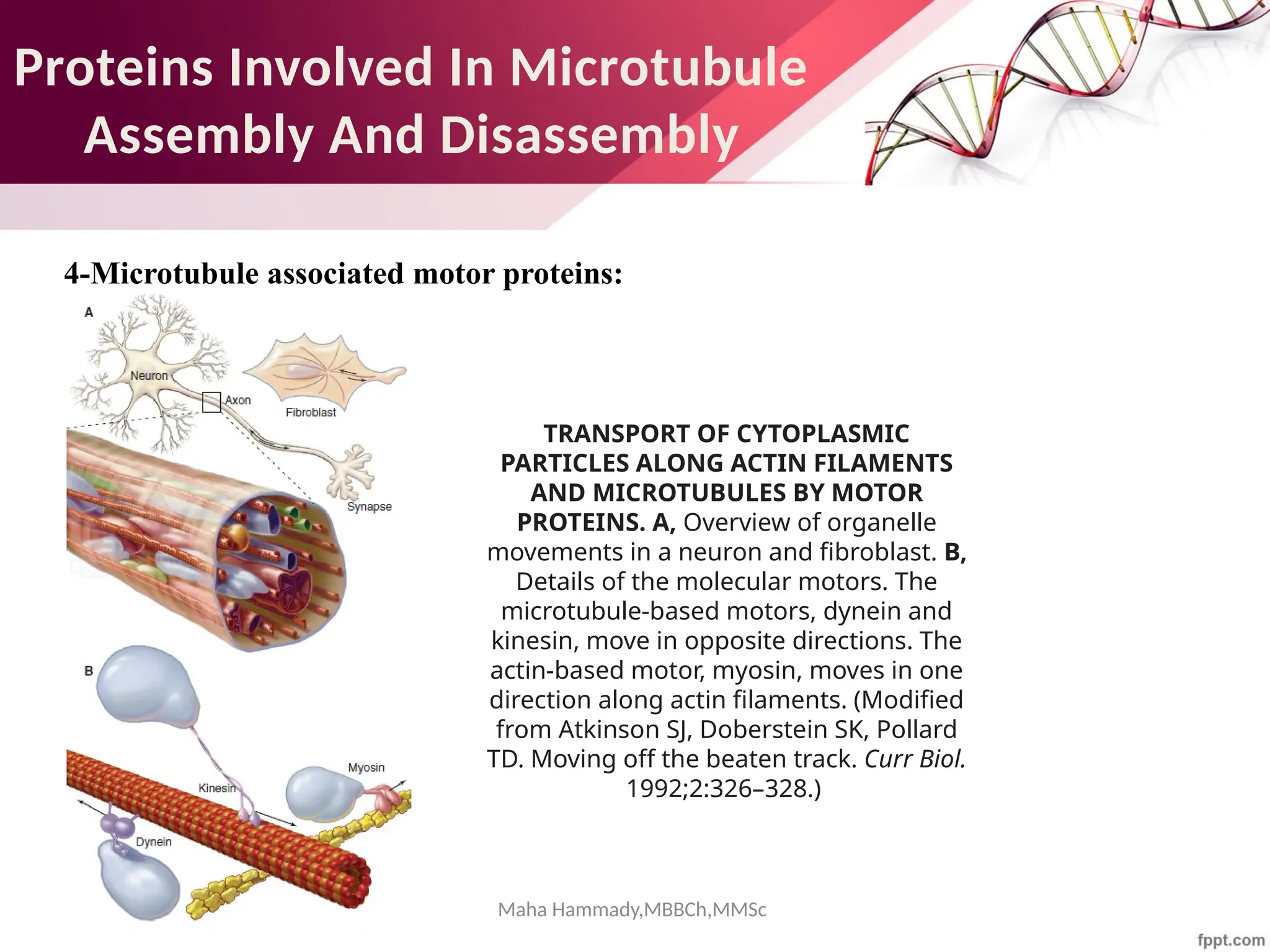
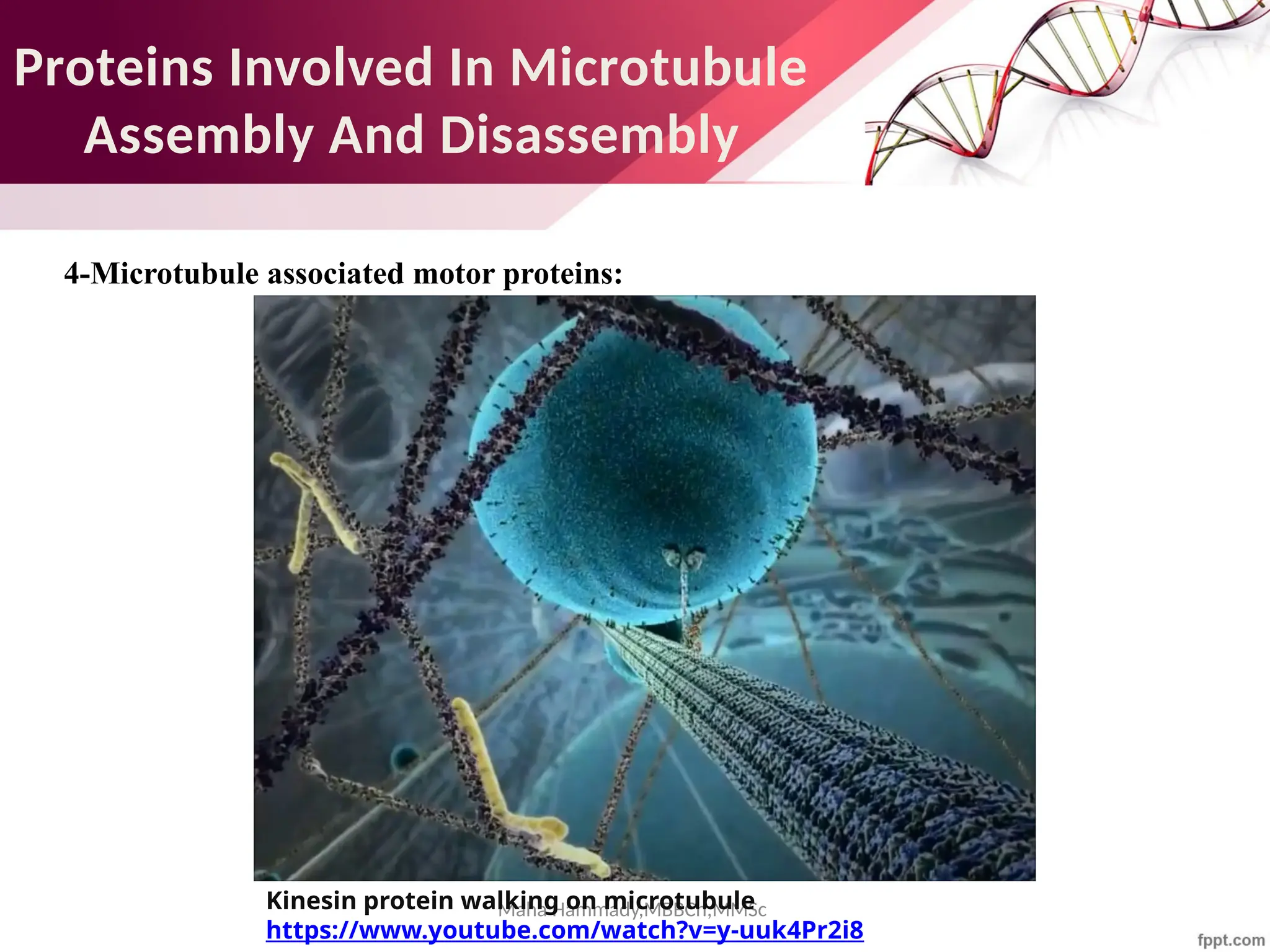
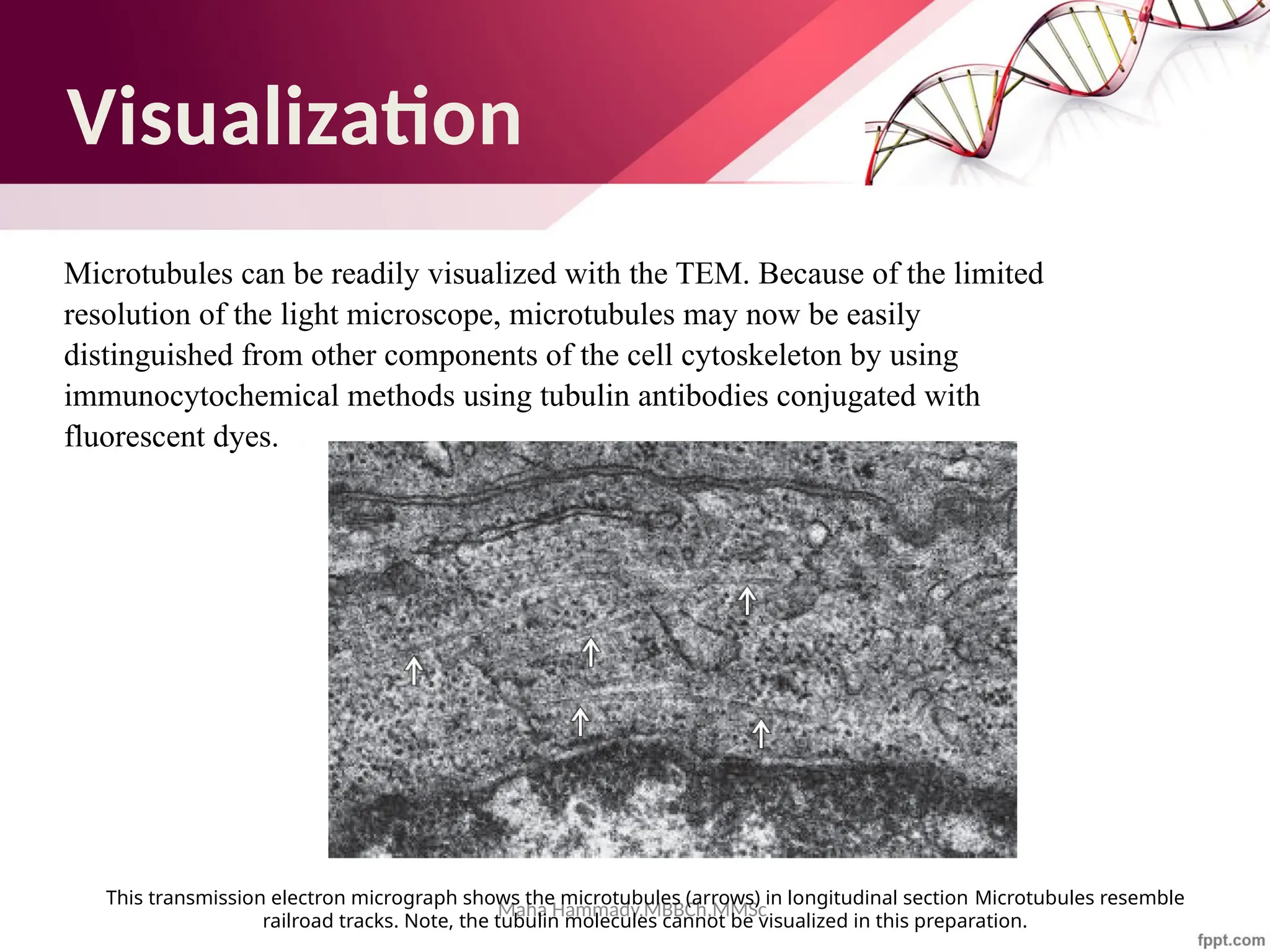
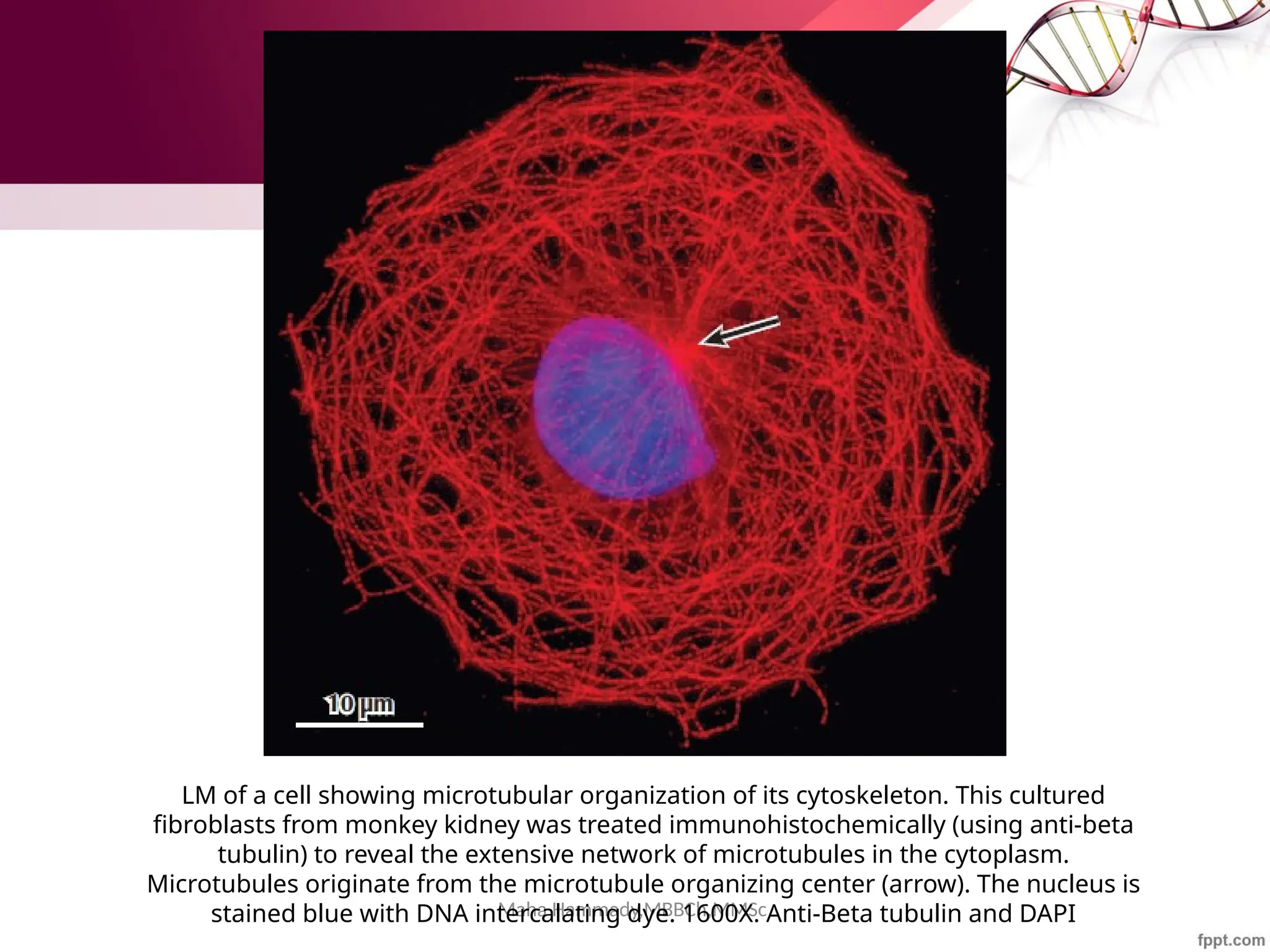
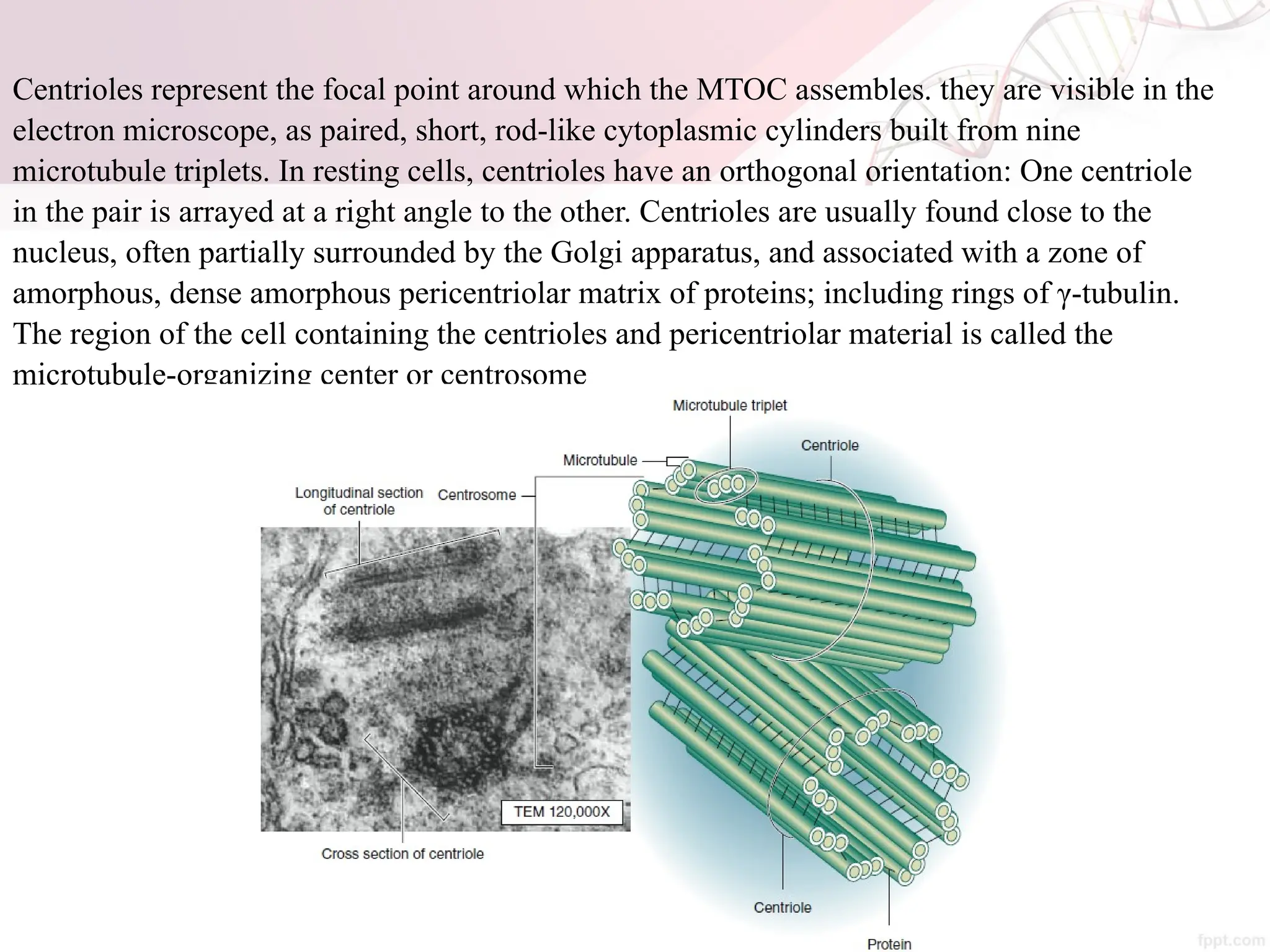
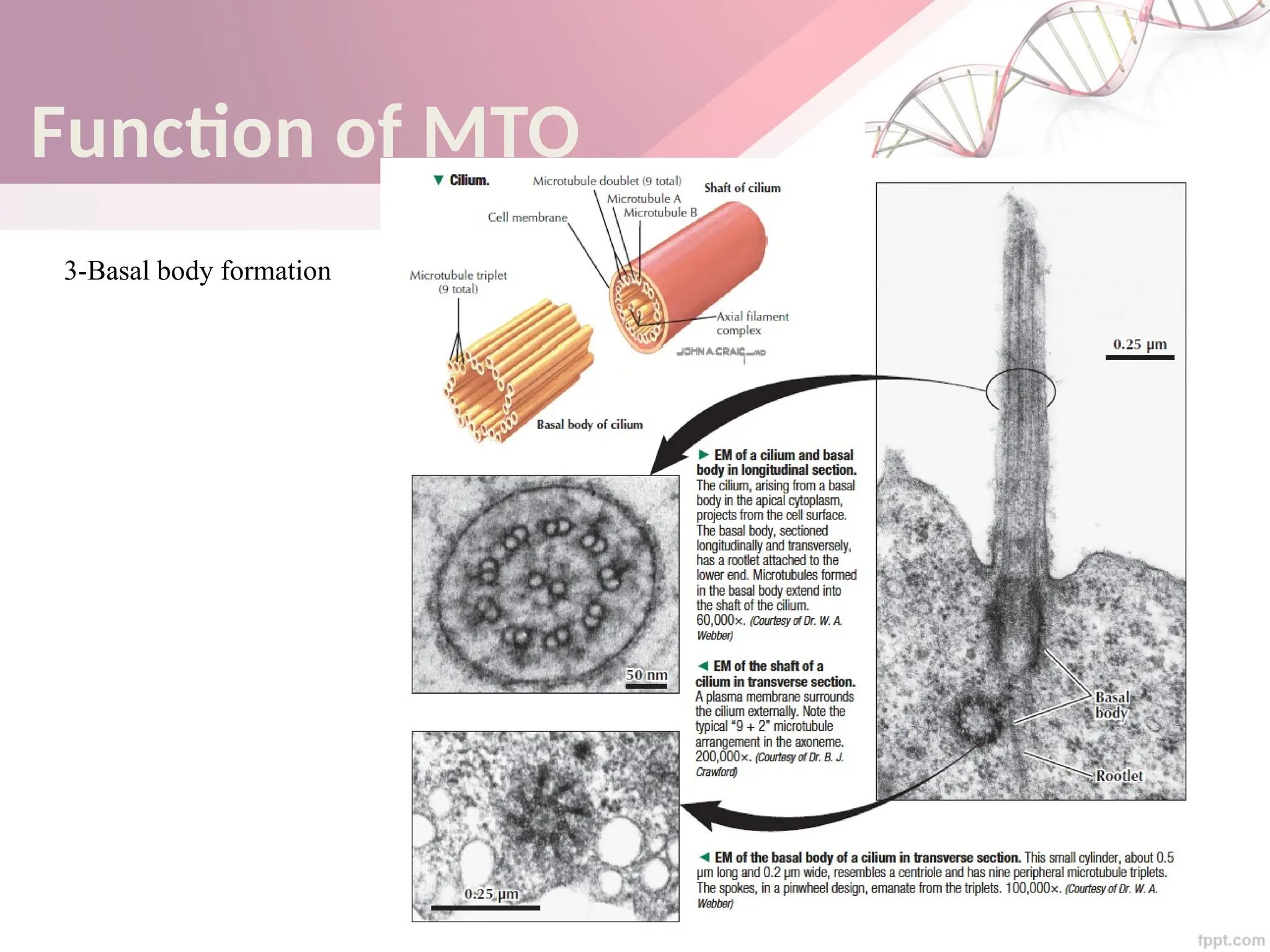
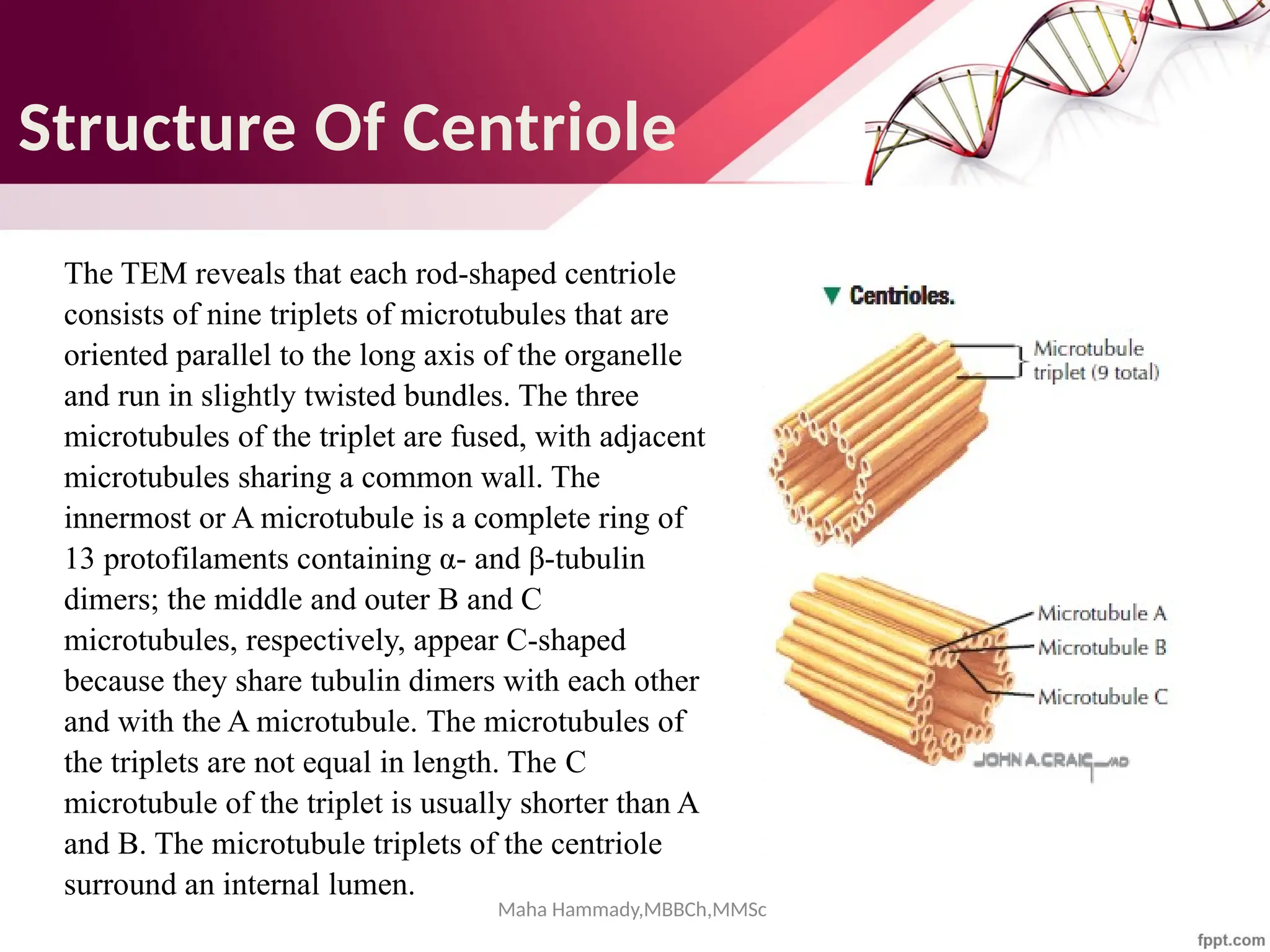
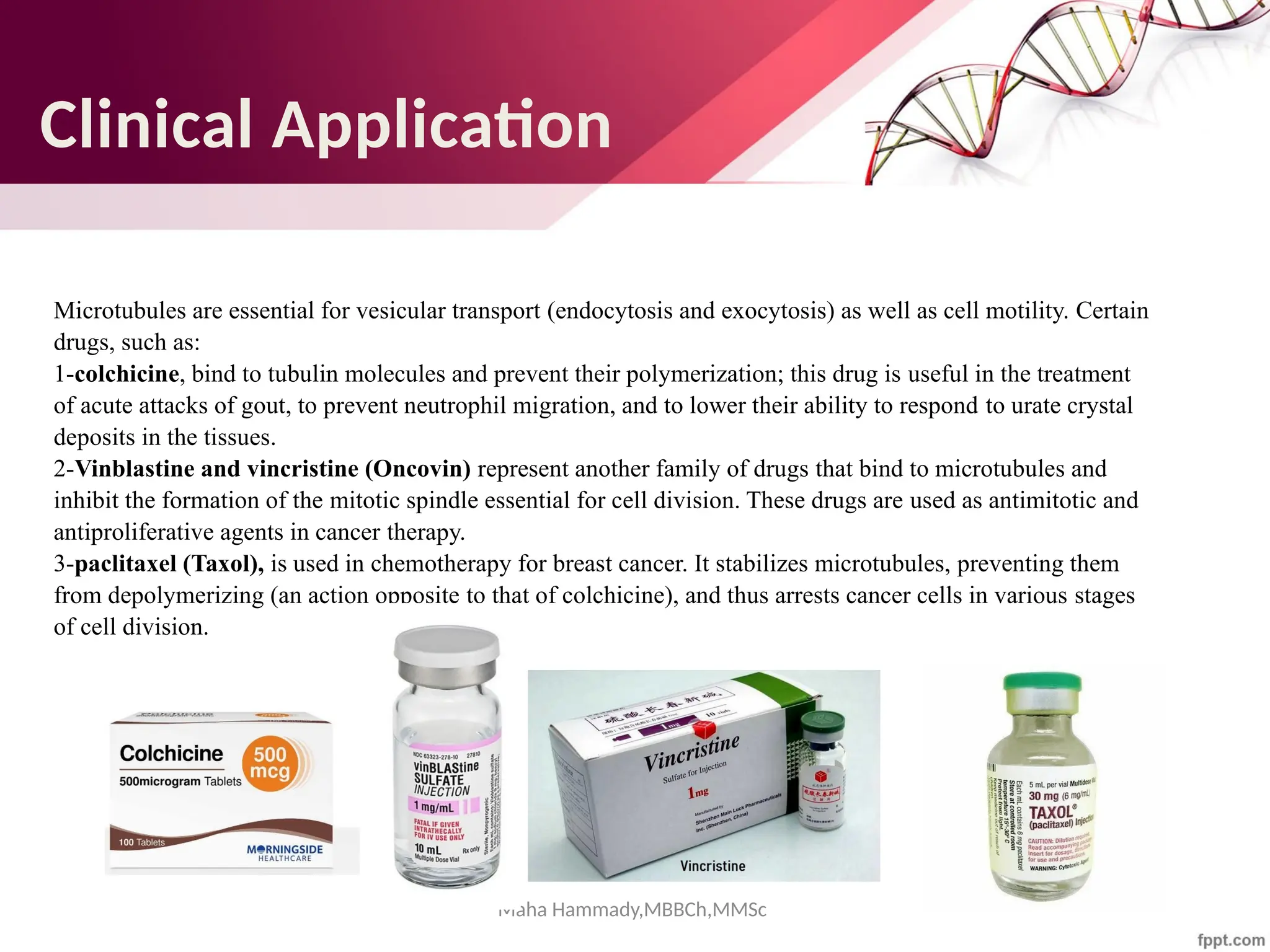
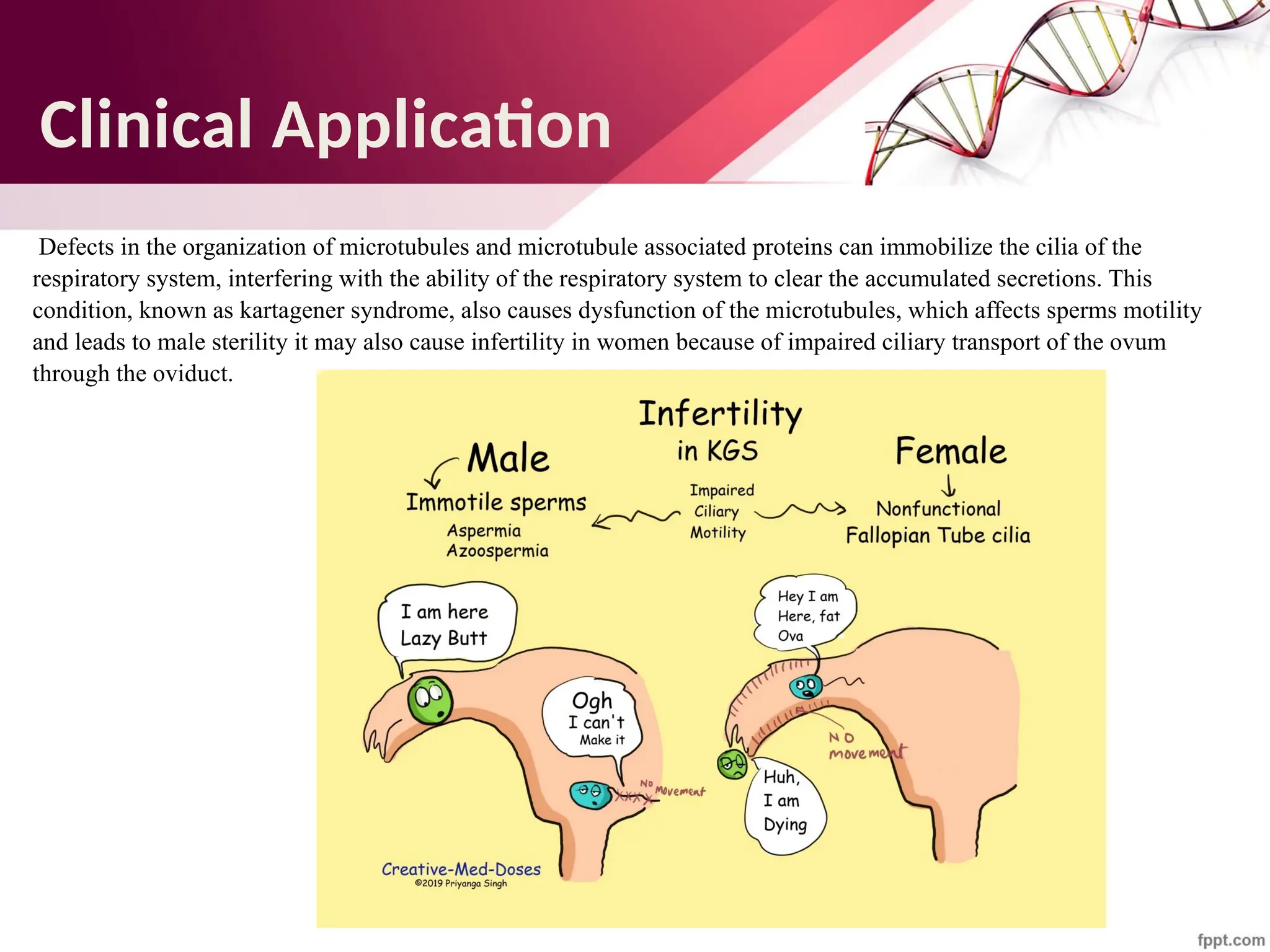
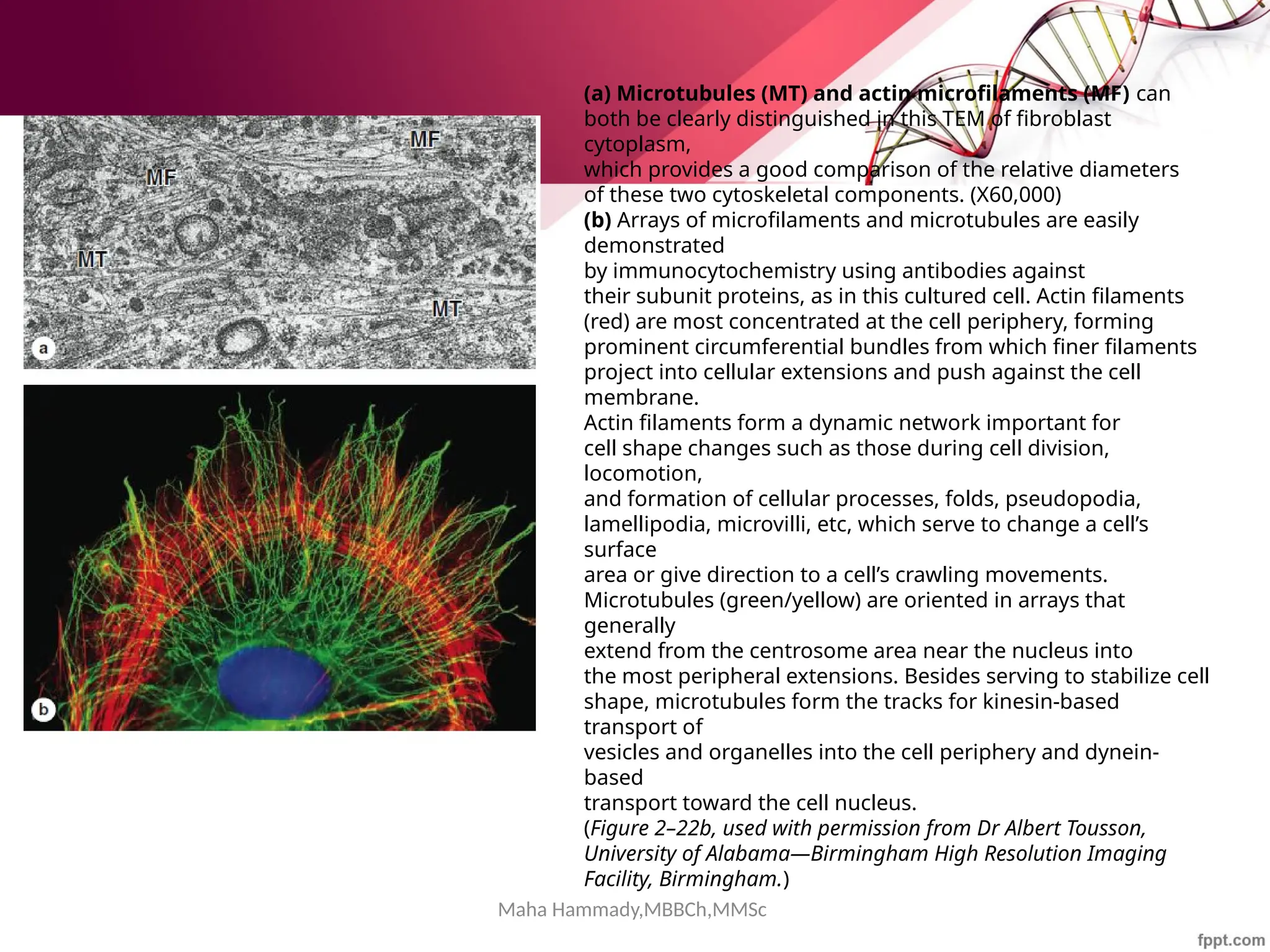
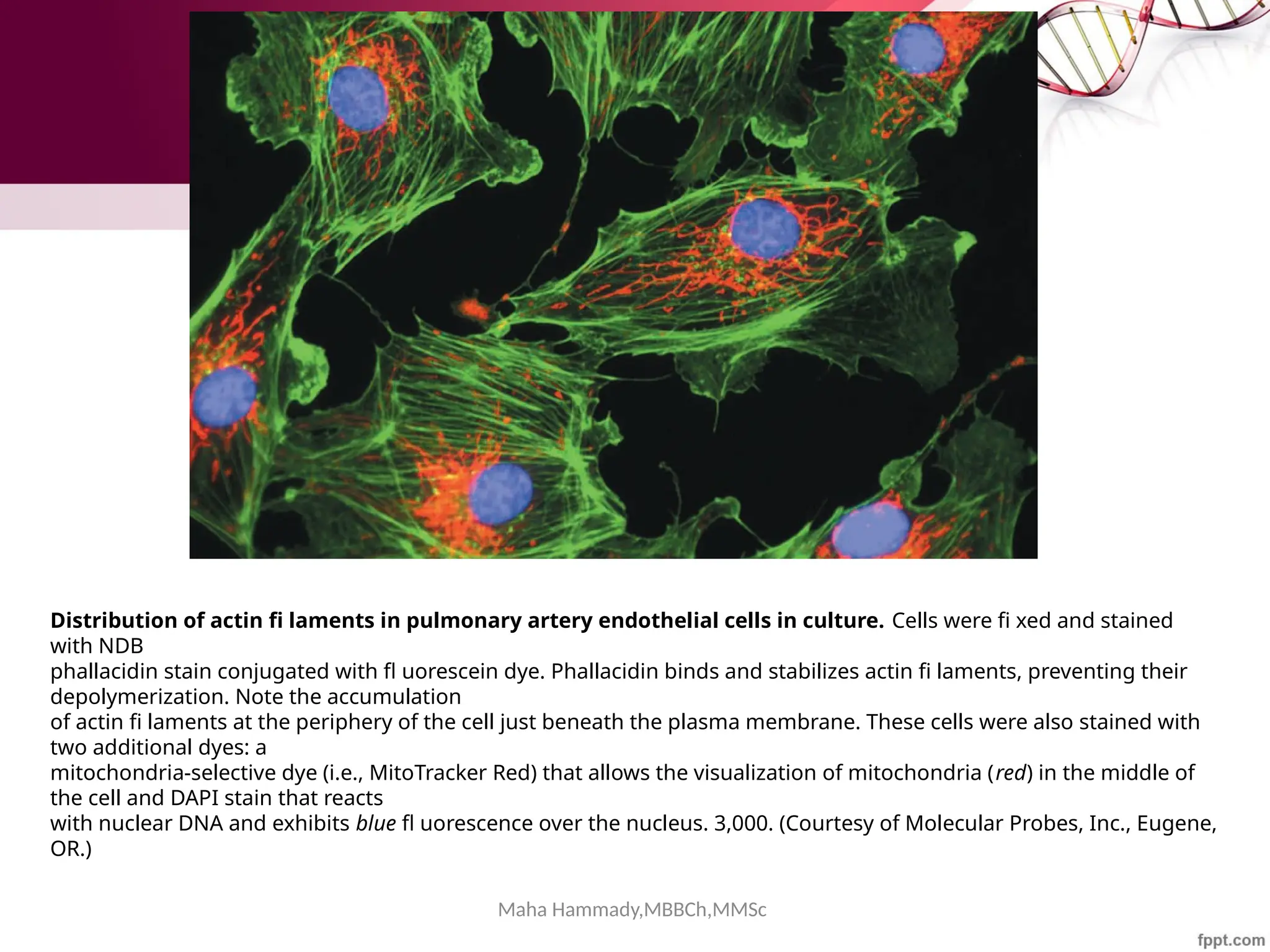
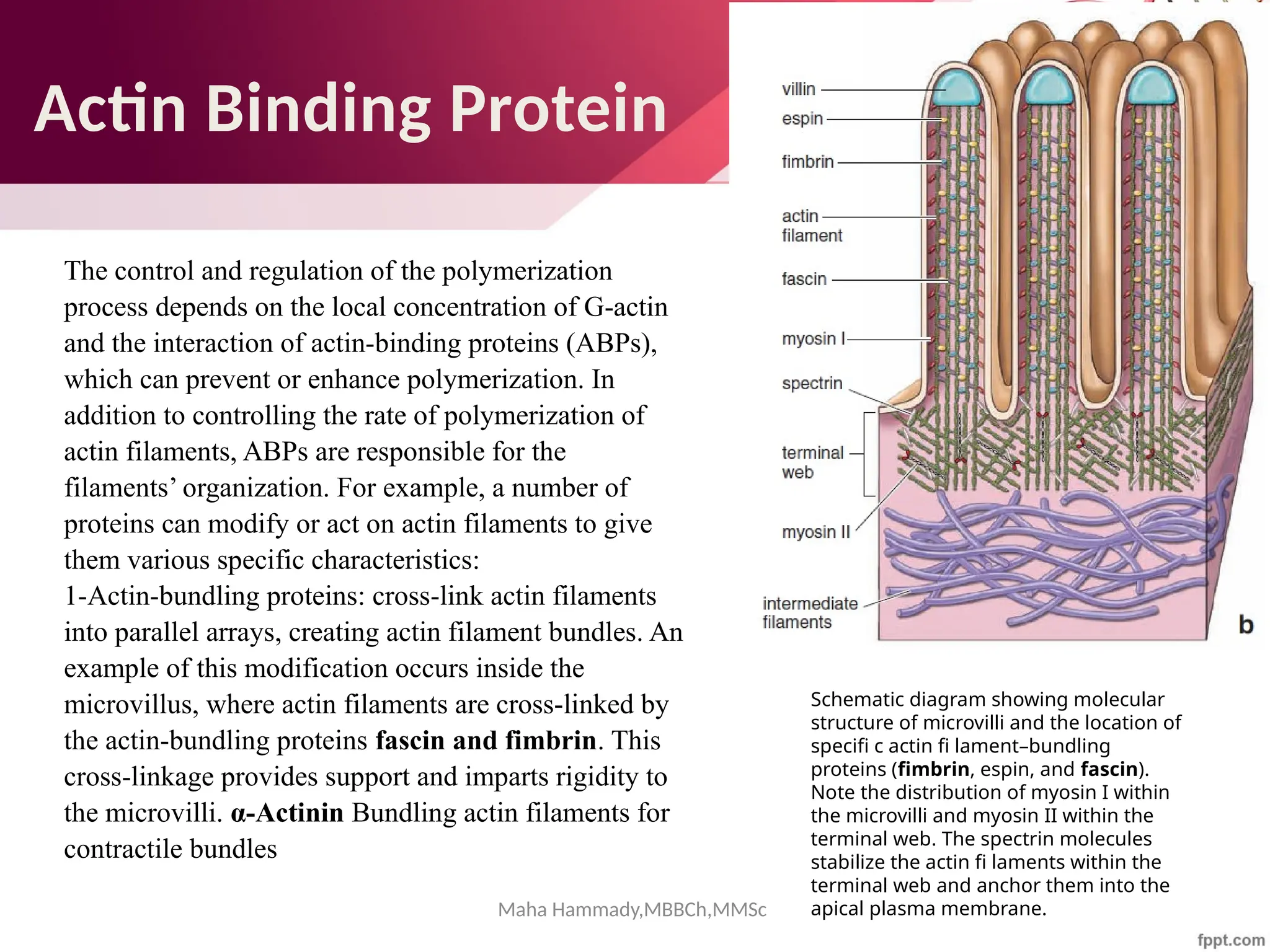
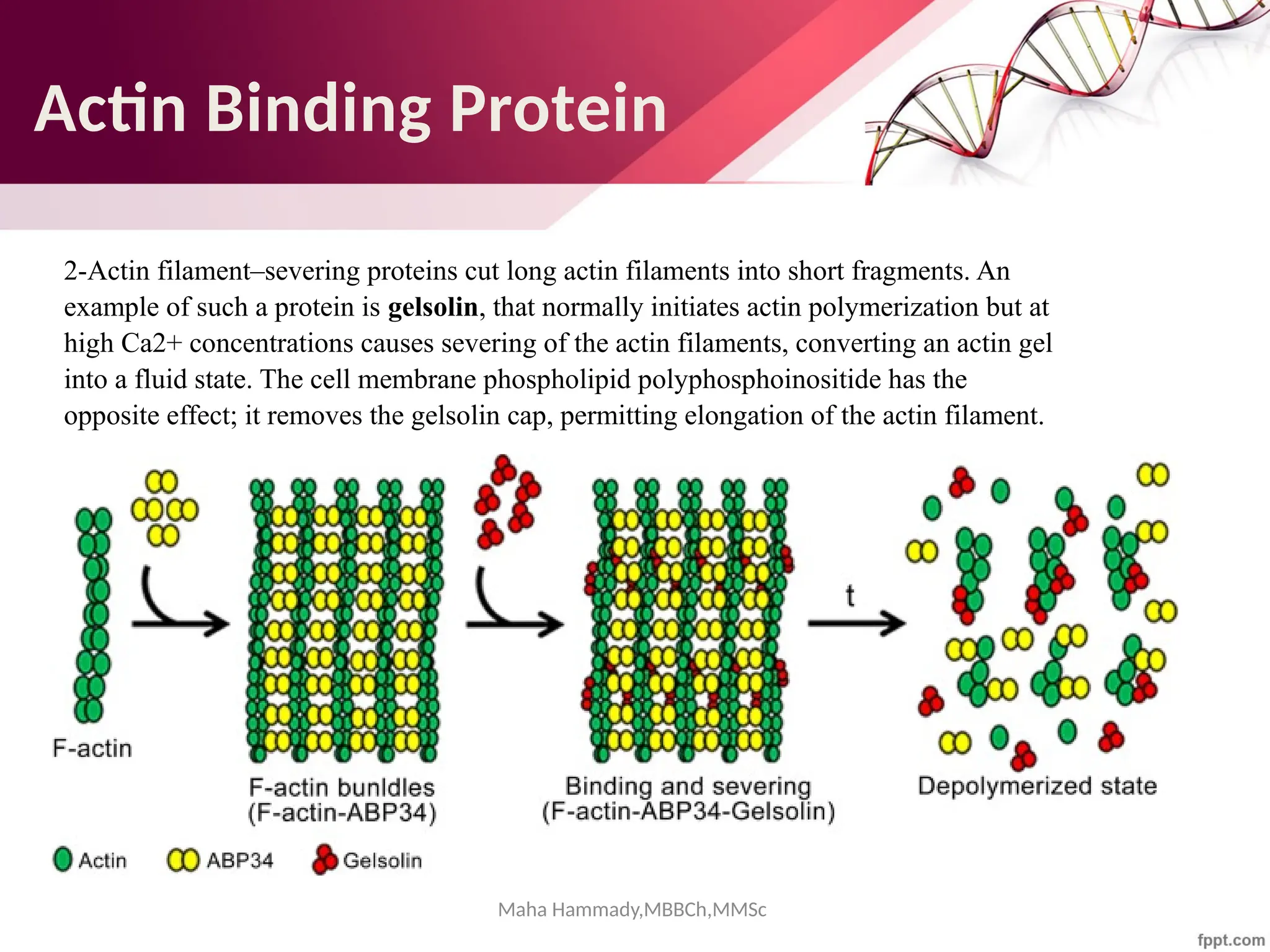
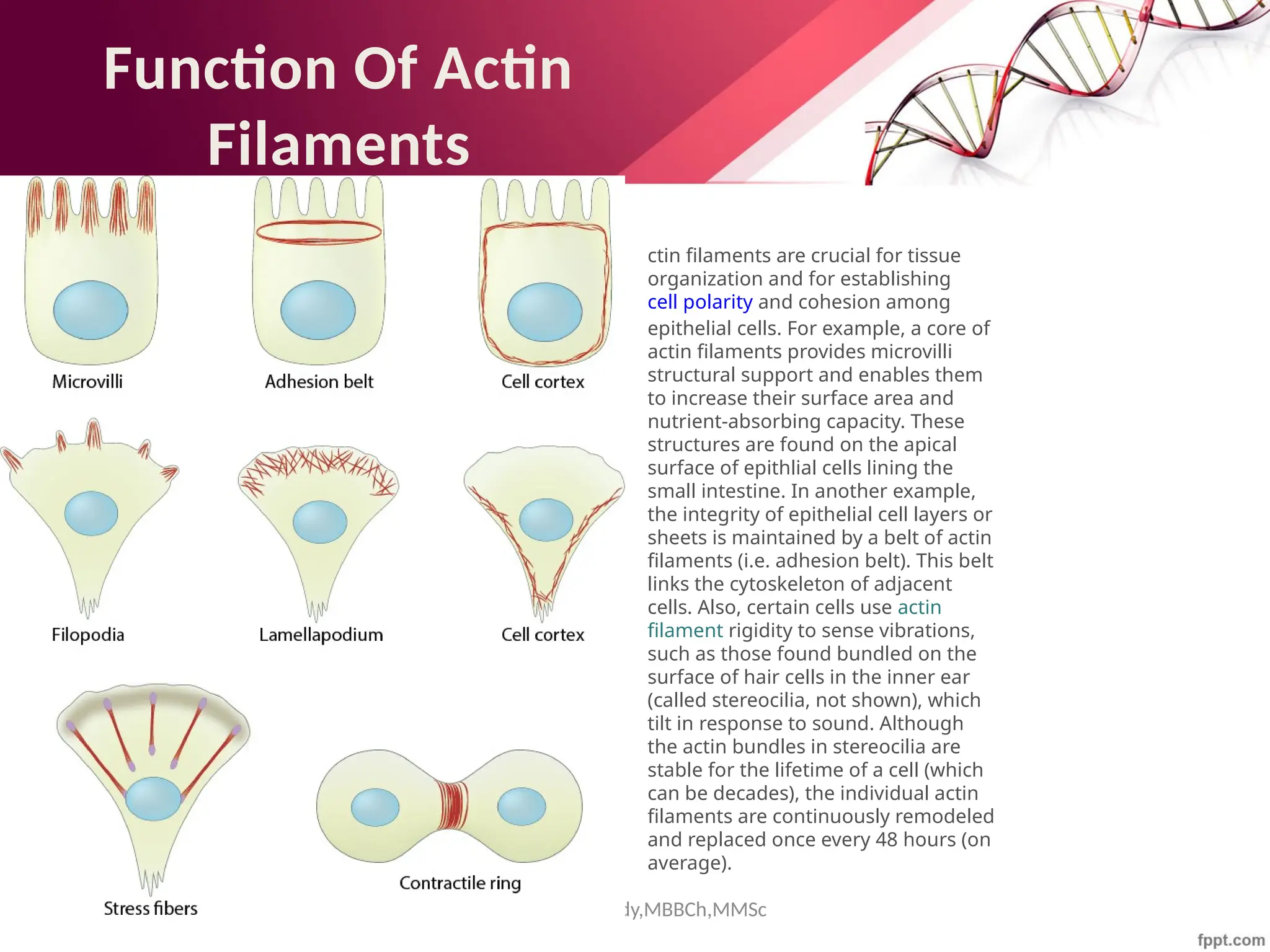
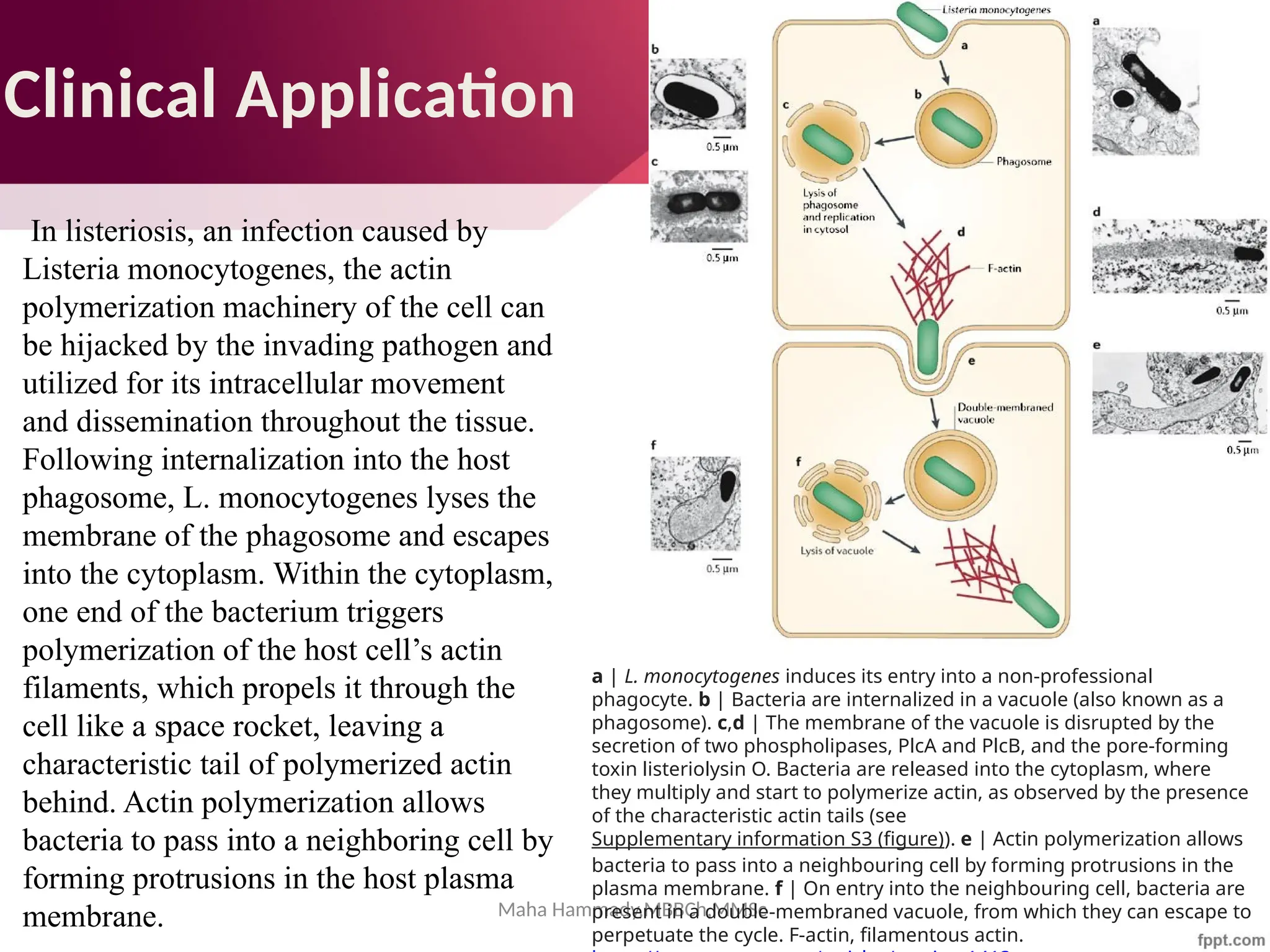
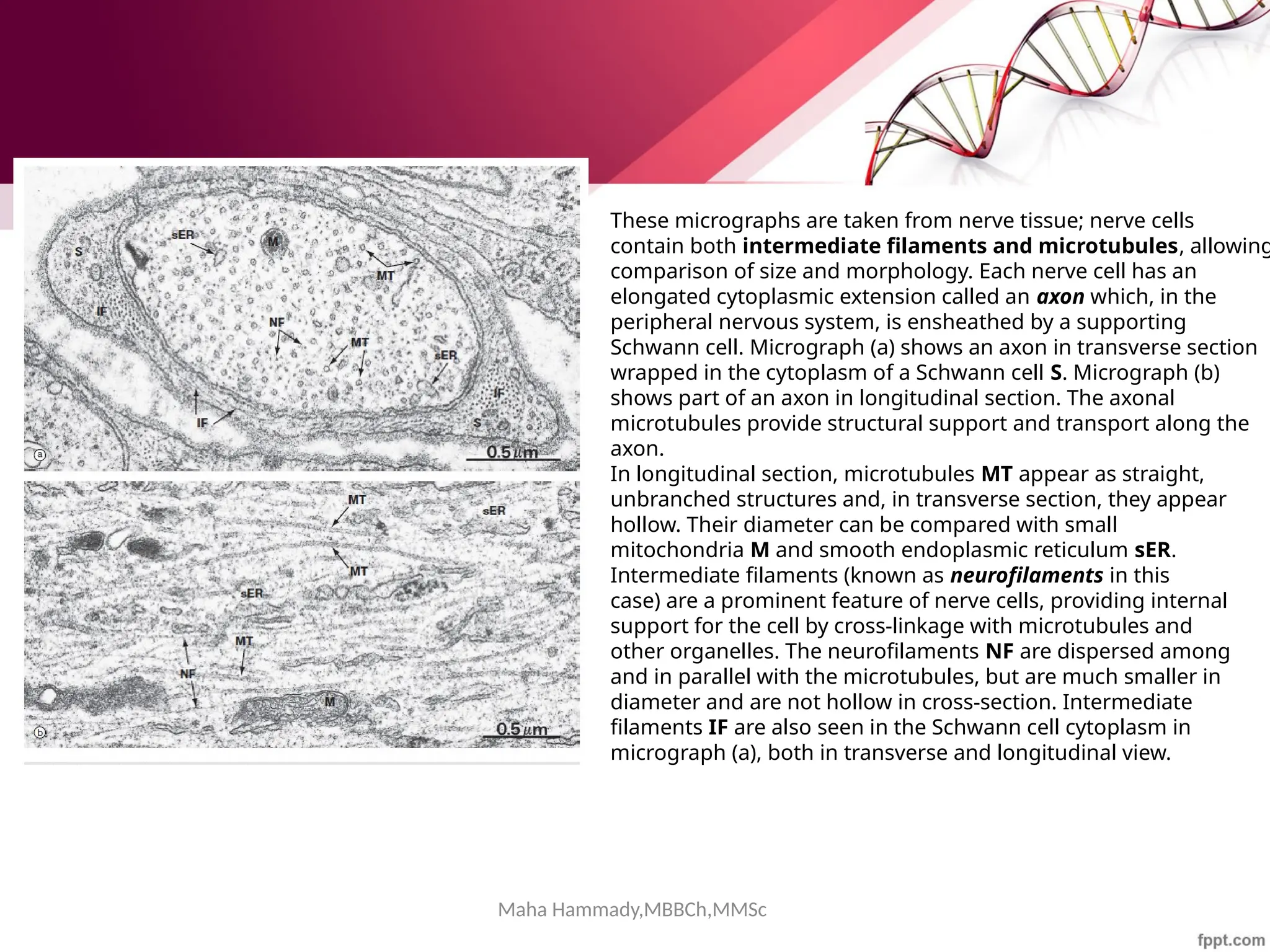
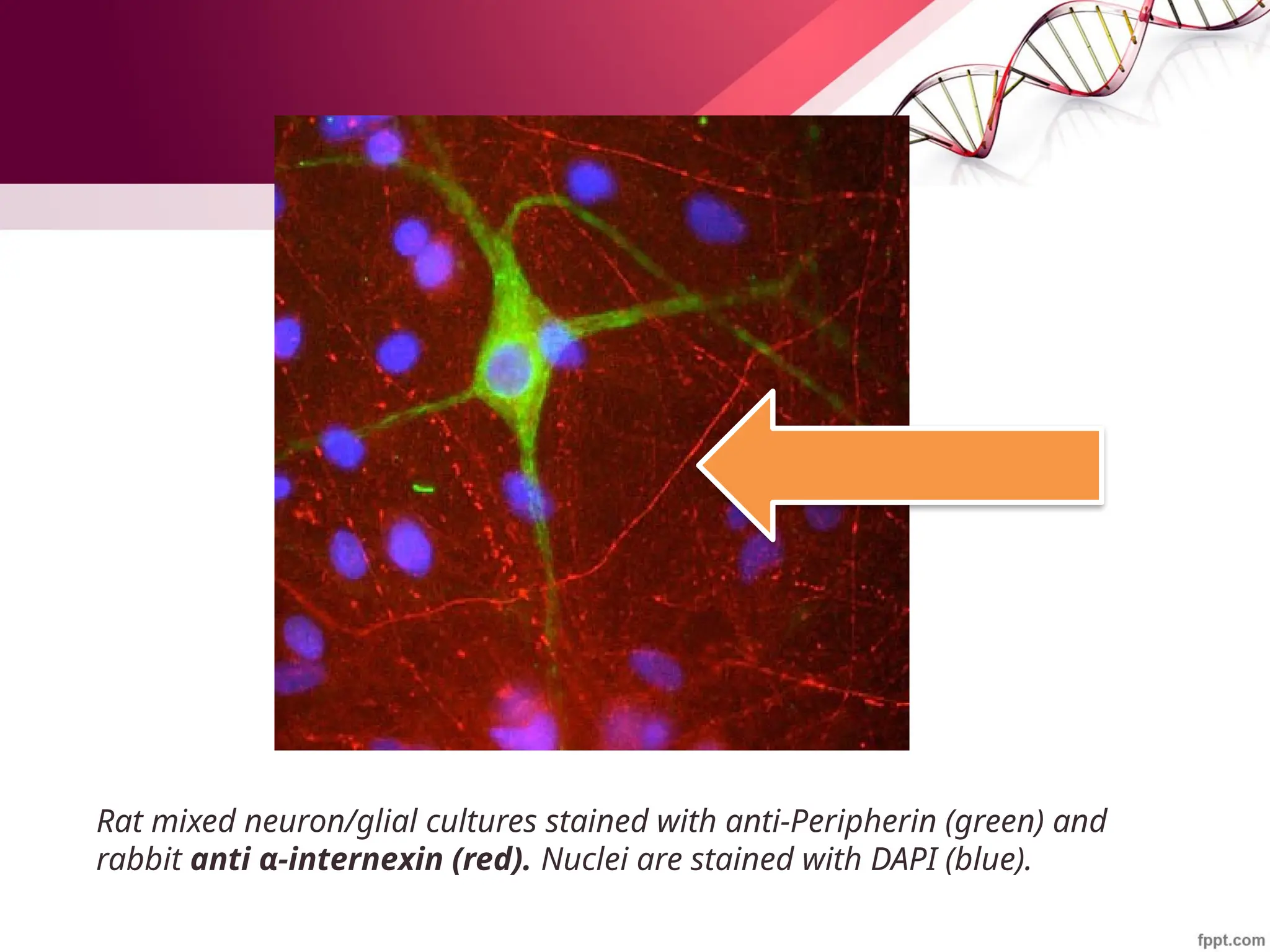
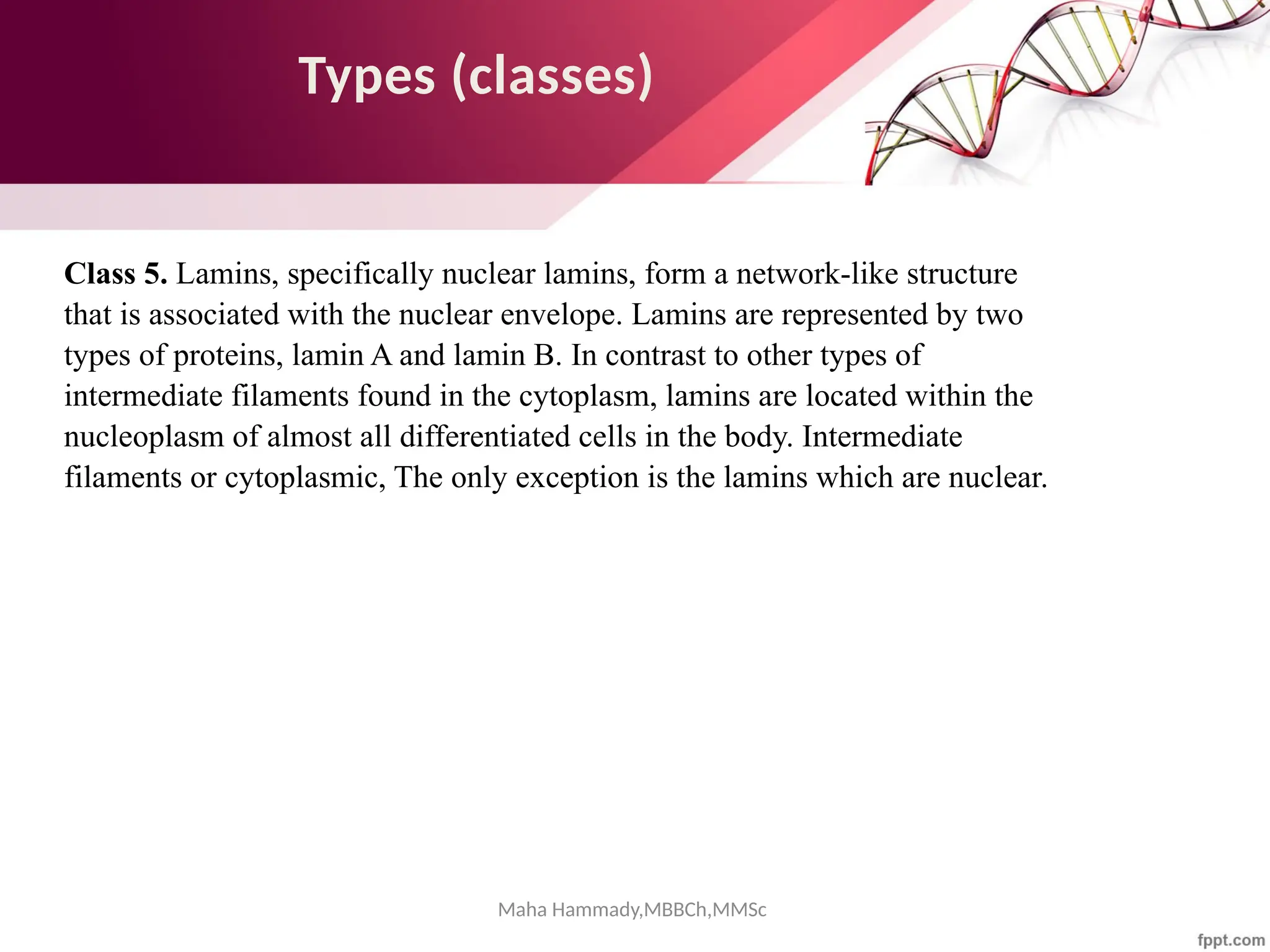
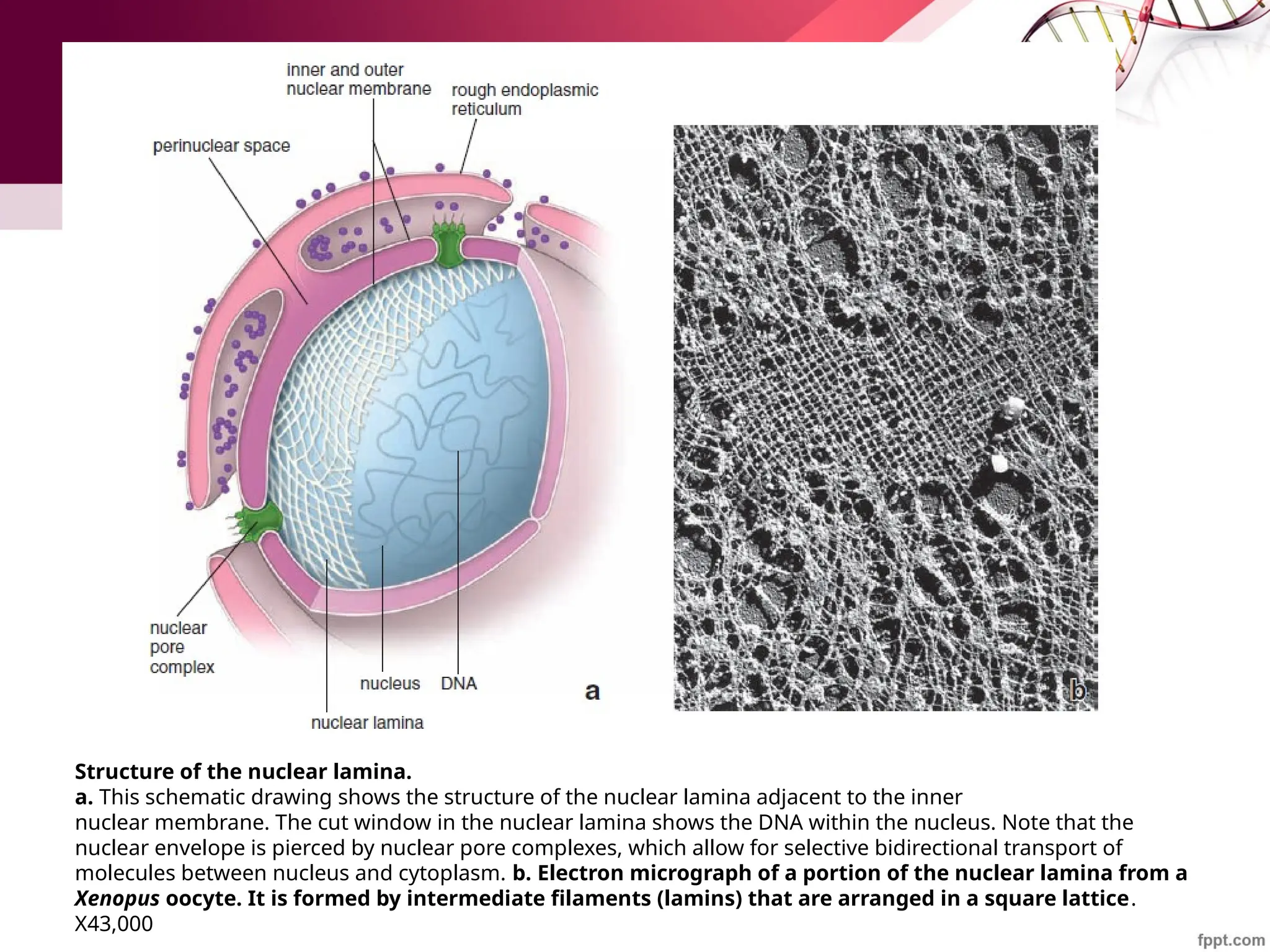
The document discusses the cytoskeleton of animal cells, detailing the microtubules' structure, function, and dynamic instability. Microtubules, comprising polymerized tubulin, play crucial roles in cellular movement, shape maintenance, and intracellular transport. It also highlights the involvement of microtubule-associated proteins (MAPs) and molecular motors in regulating microtubule dynamics and functioning within neuronal structures.






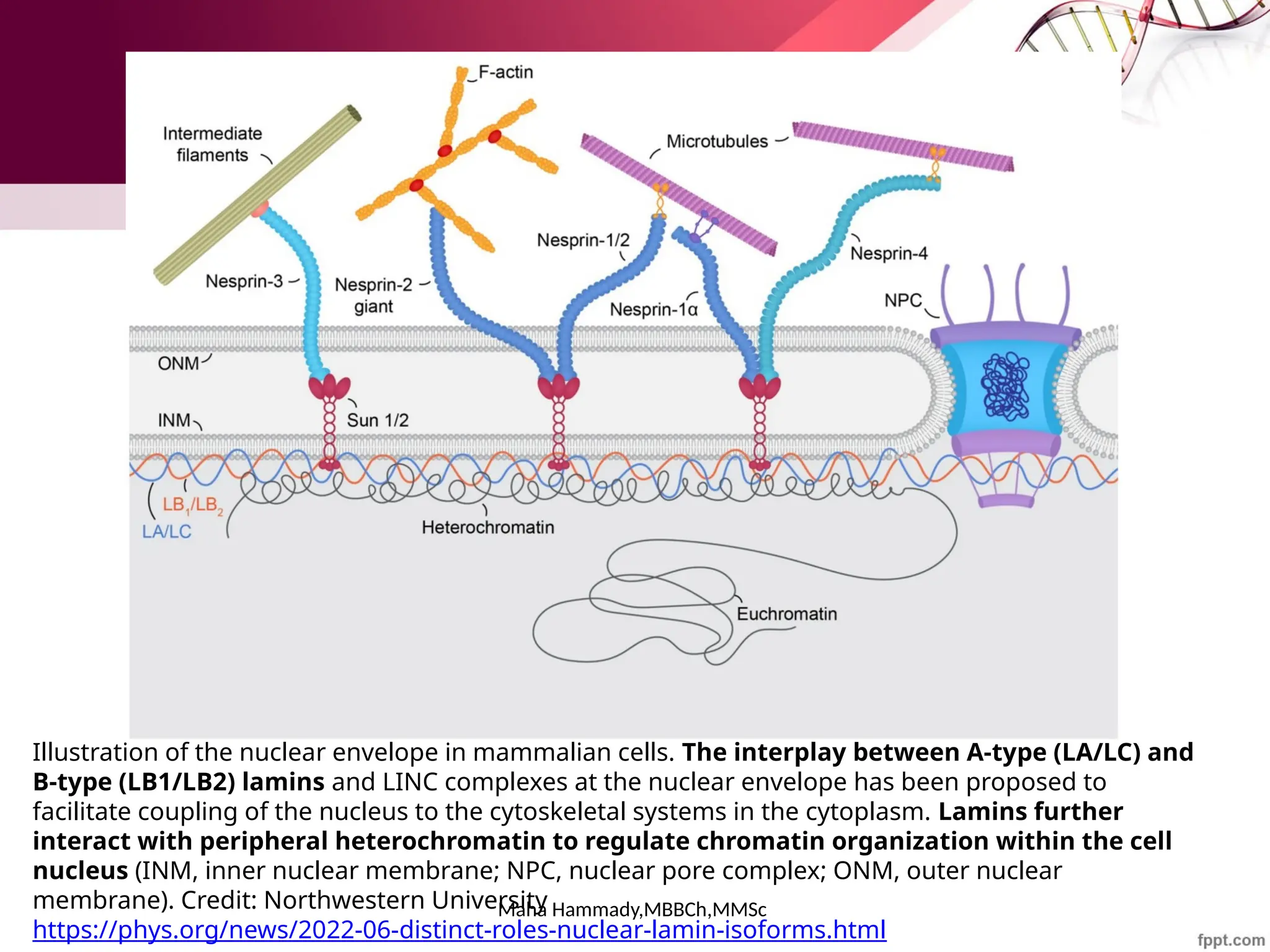
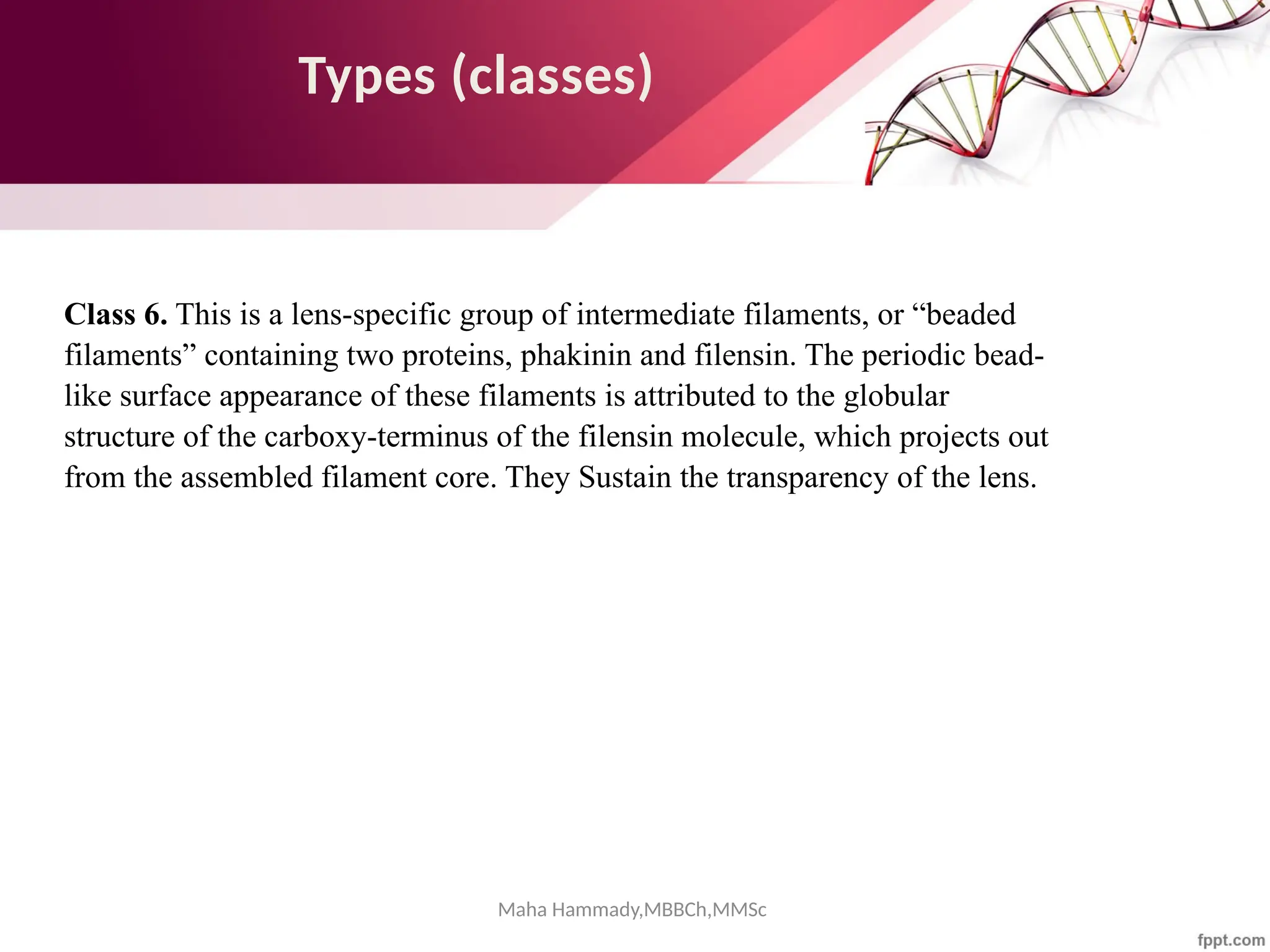
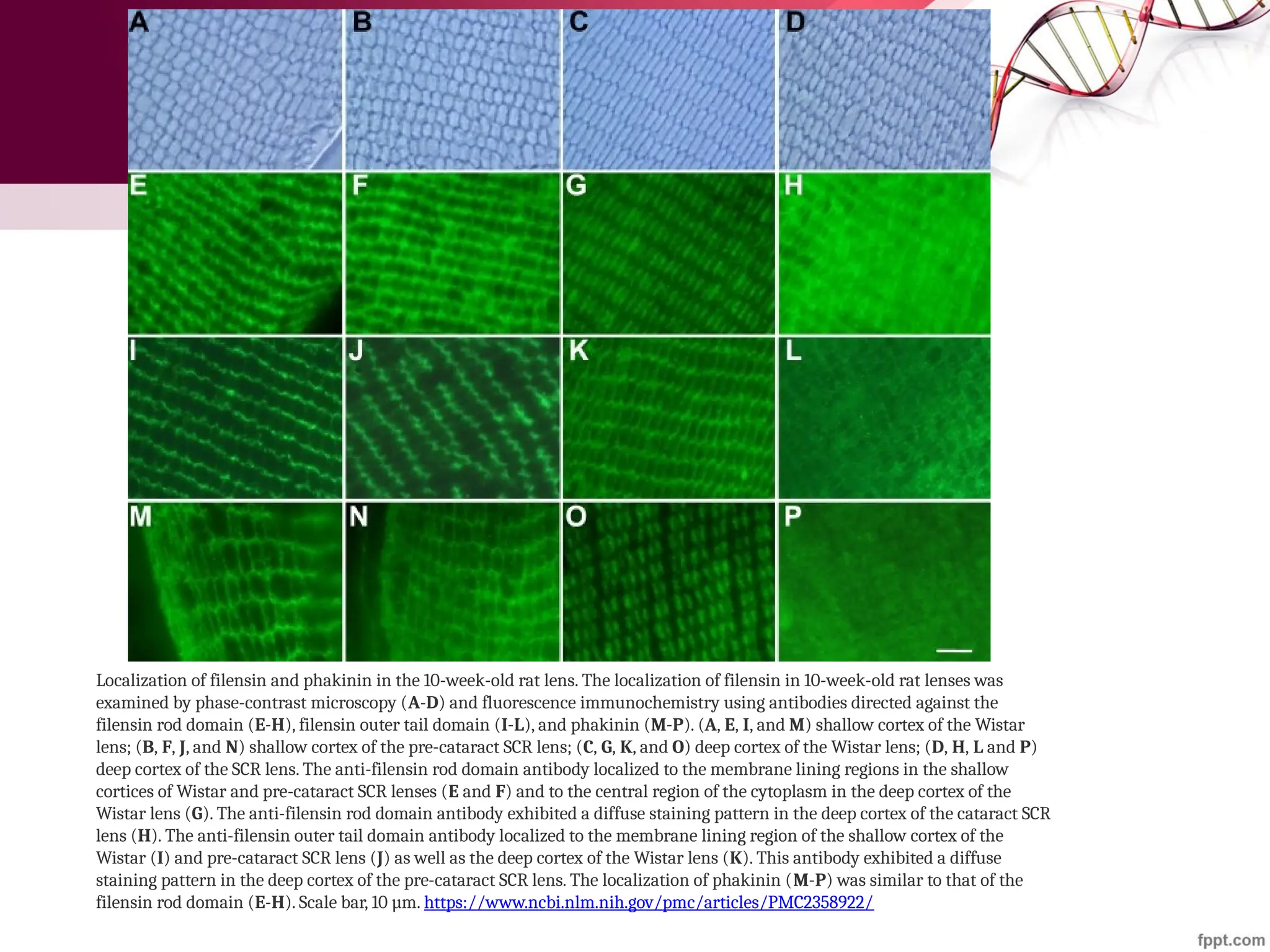
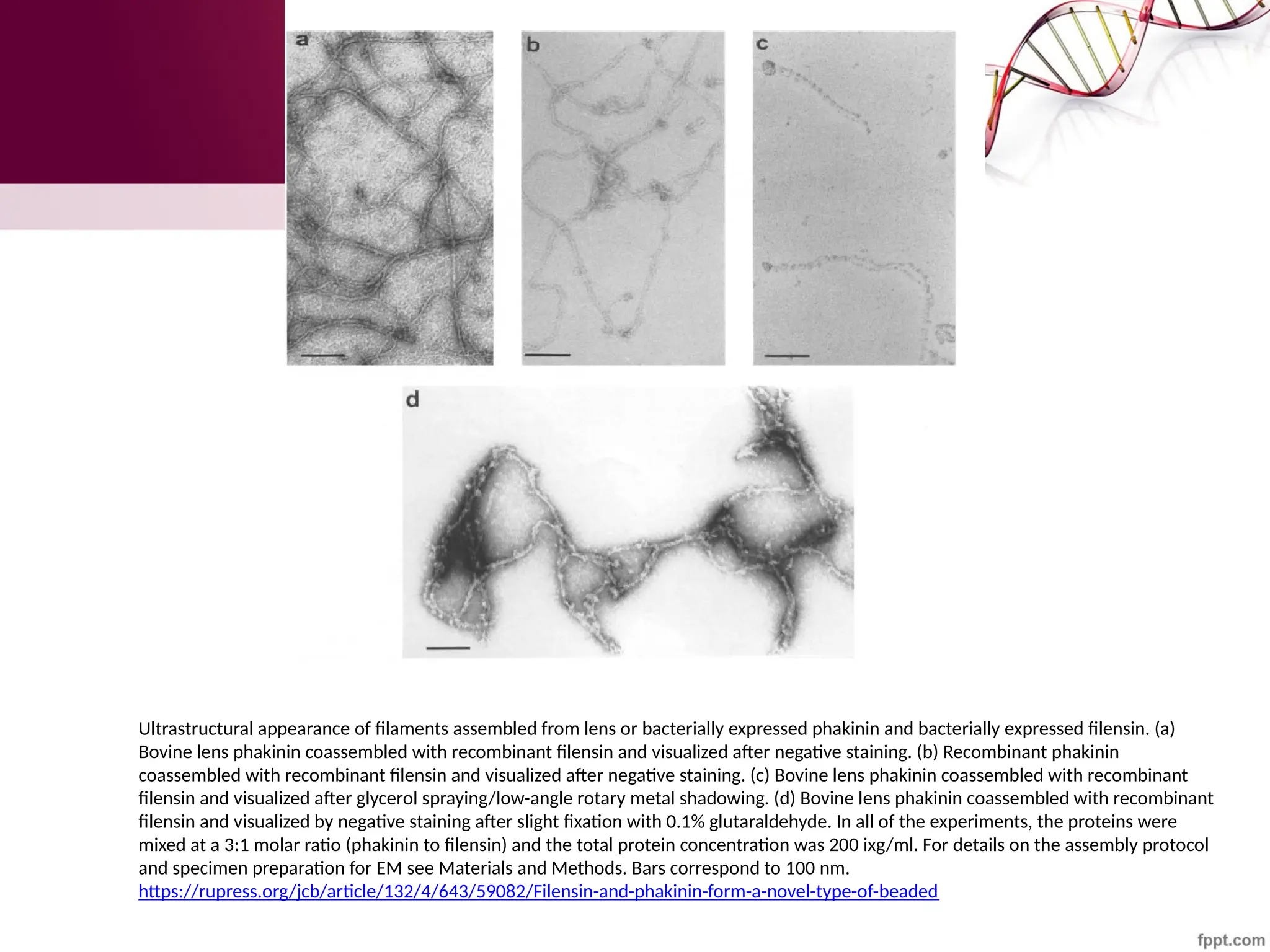
![Maha Hammady,MBBCh,MMSc
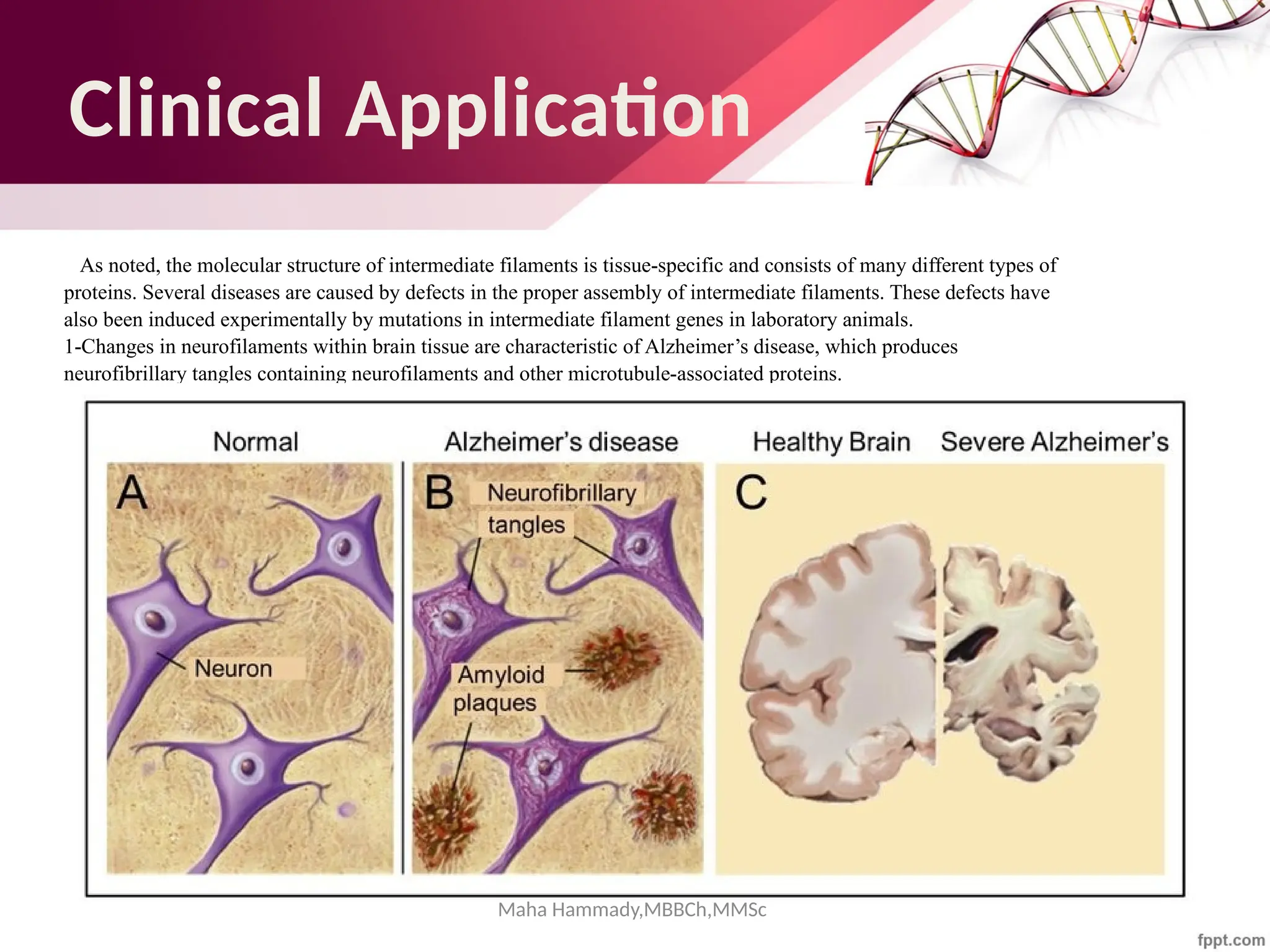
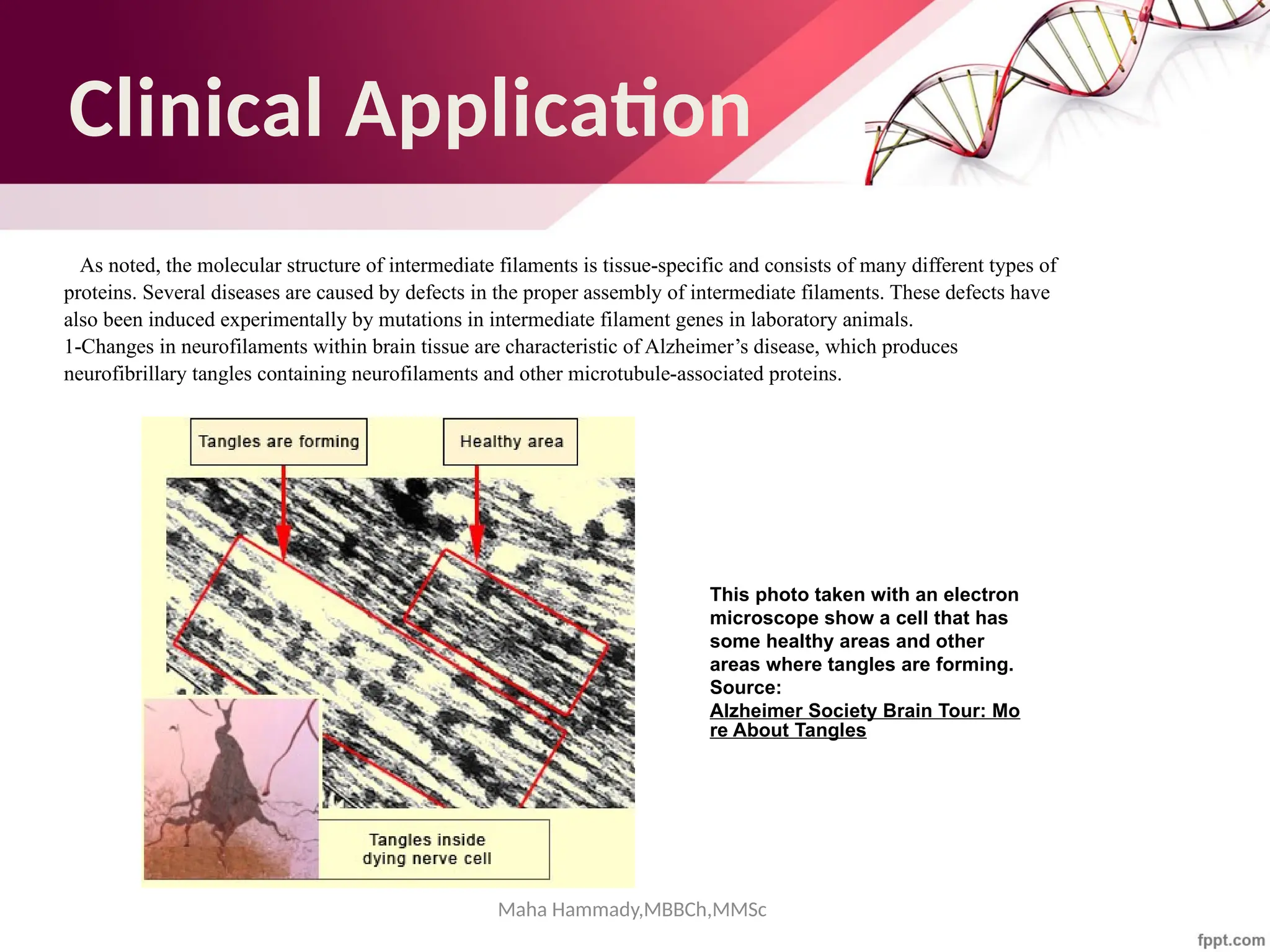
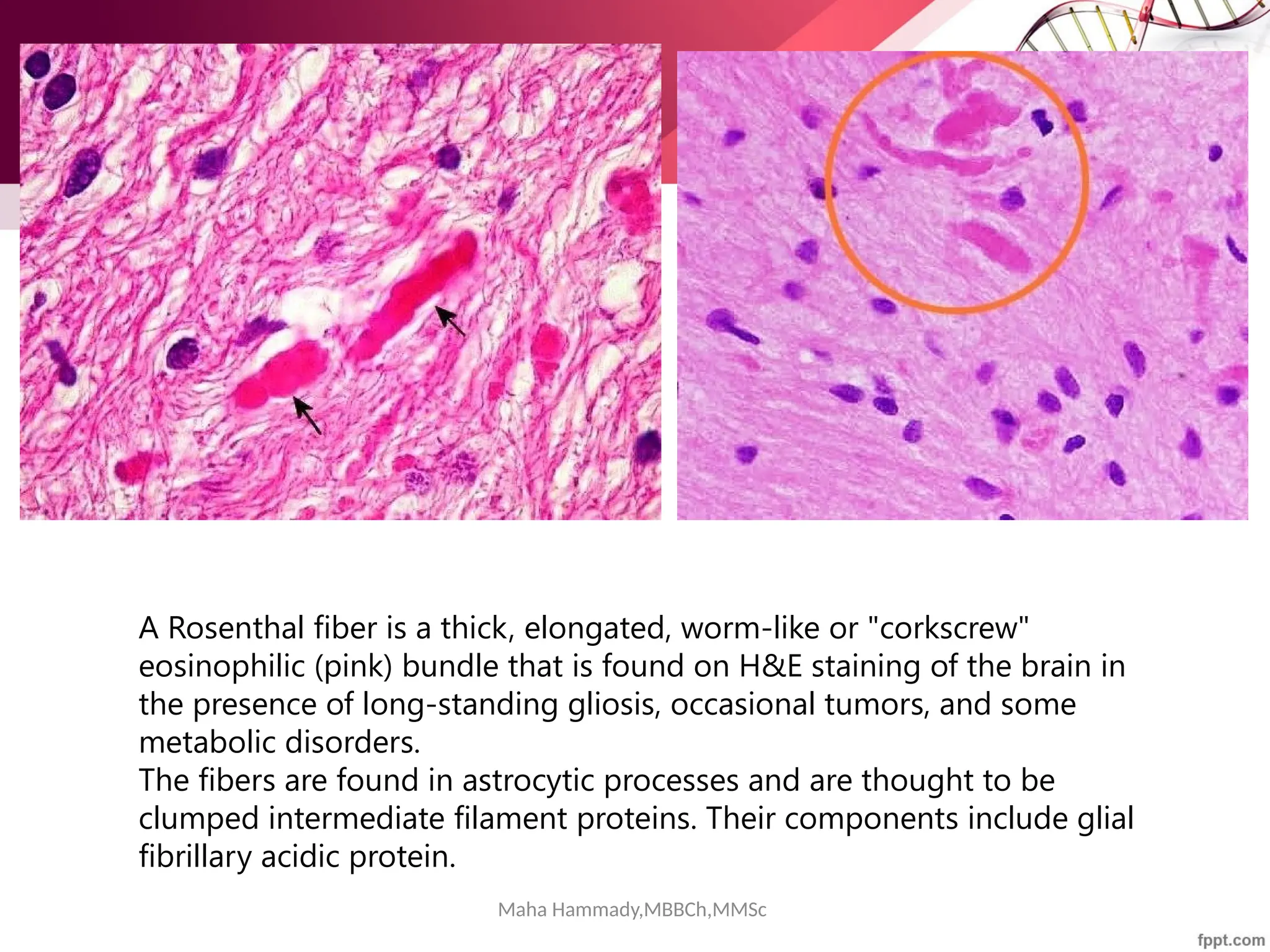
Photomicrograph from a pilocytic astrocytoma showing brightly staining red Rosenthal
fibers (arrowheads). (Hematoxylin-eosin [H&E]; original magnification, 1000×
https://www.ajnr.org/content/27/5/958](https://image.slidesharecdn.com/cytoskeleton-mahahammady-241025124340-0e8d3e44/75/The-Cytoskeleton-Structure-Function-and-Cellular-Dynamics-maha-hammady-pptx-93-2048.jpg)