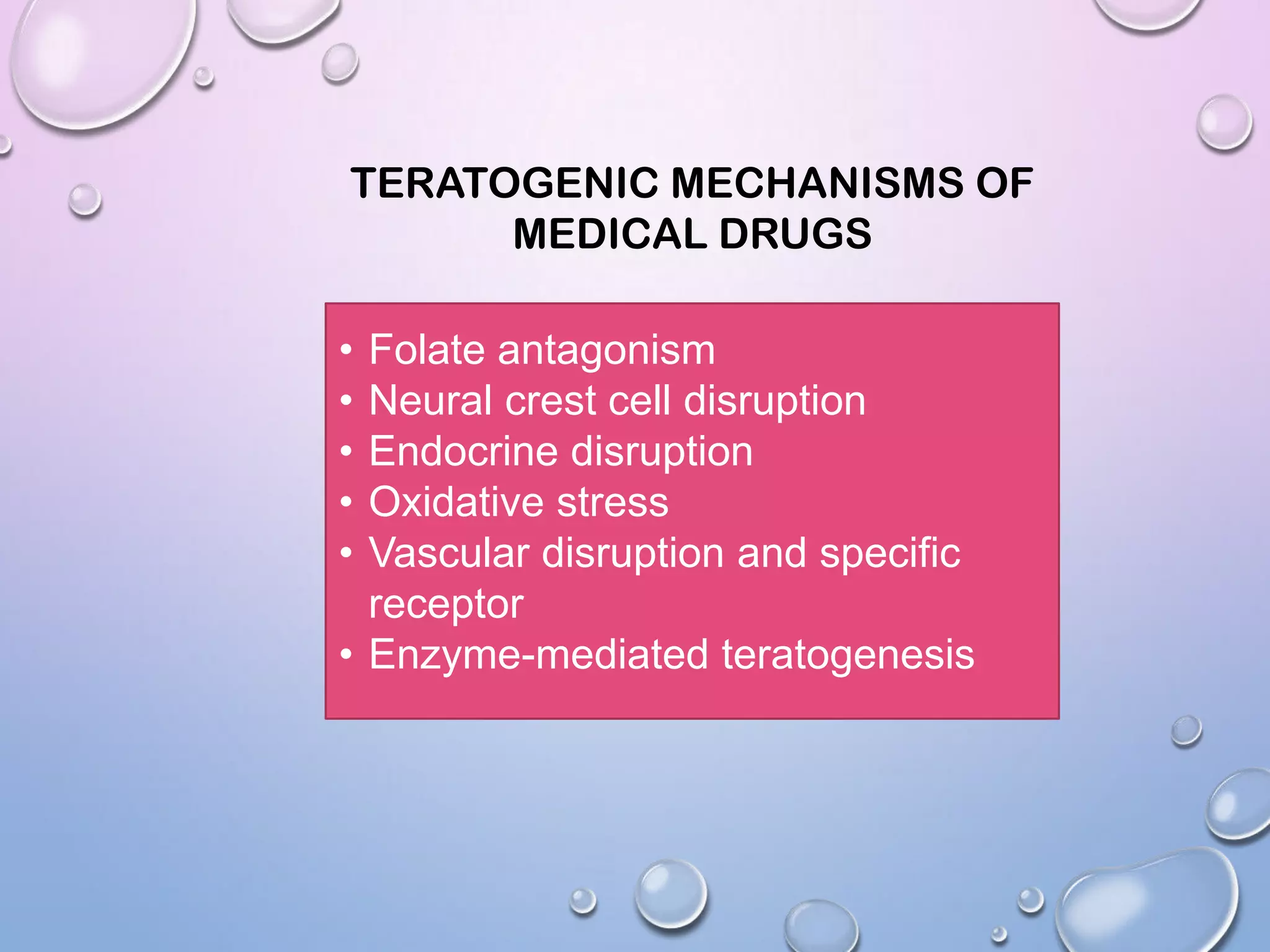
This document discusses teratogens and teratogenic drugs. It begins by defining a teratogen as an agent that can disturb fetal development. It then discusses the FDA pregnancy categories and mechanisms of action of teratogenic drugs. Specific examples of teratogenic drugs are given for different categories, along with their mechanisms and potential effects on the fetus. The document stresses the importance of carefully considering drug use during pregnancy and providing counseling on risks and benefits.