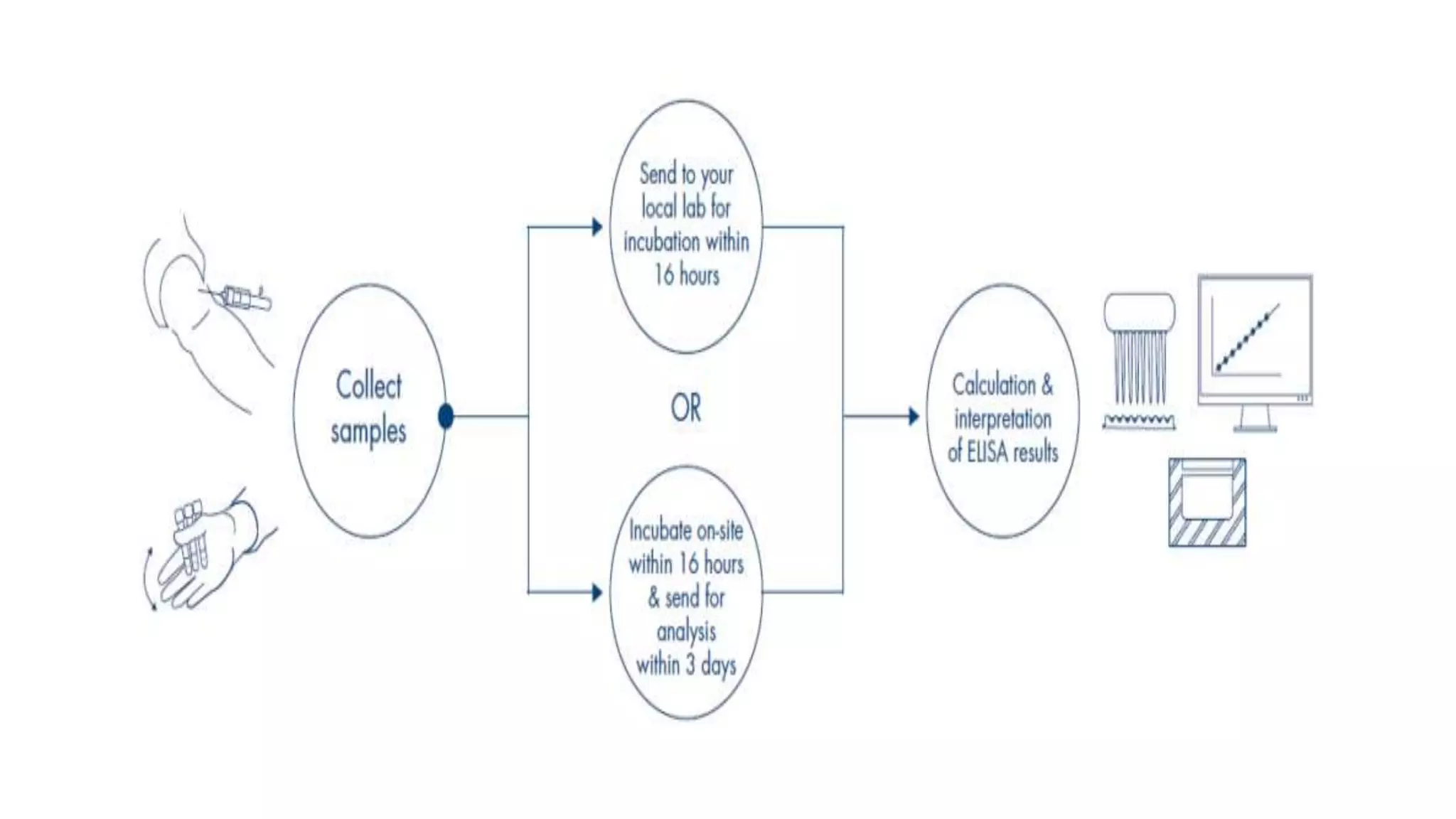
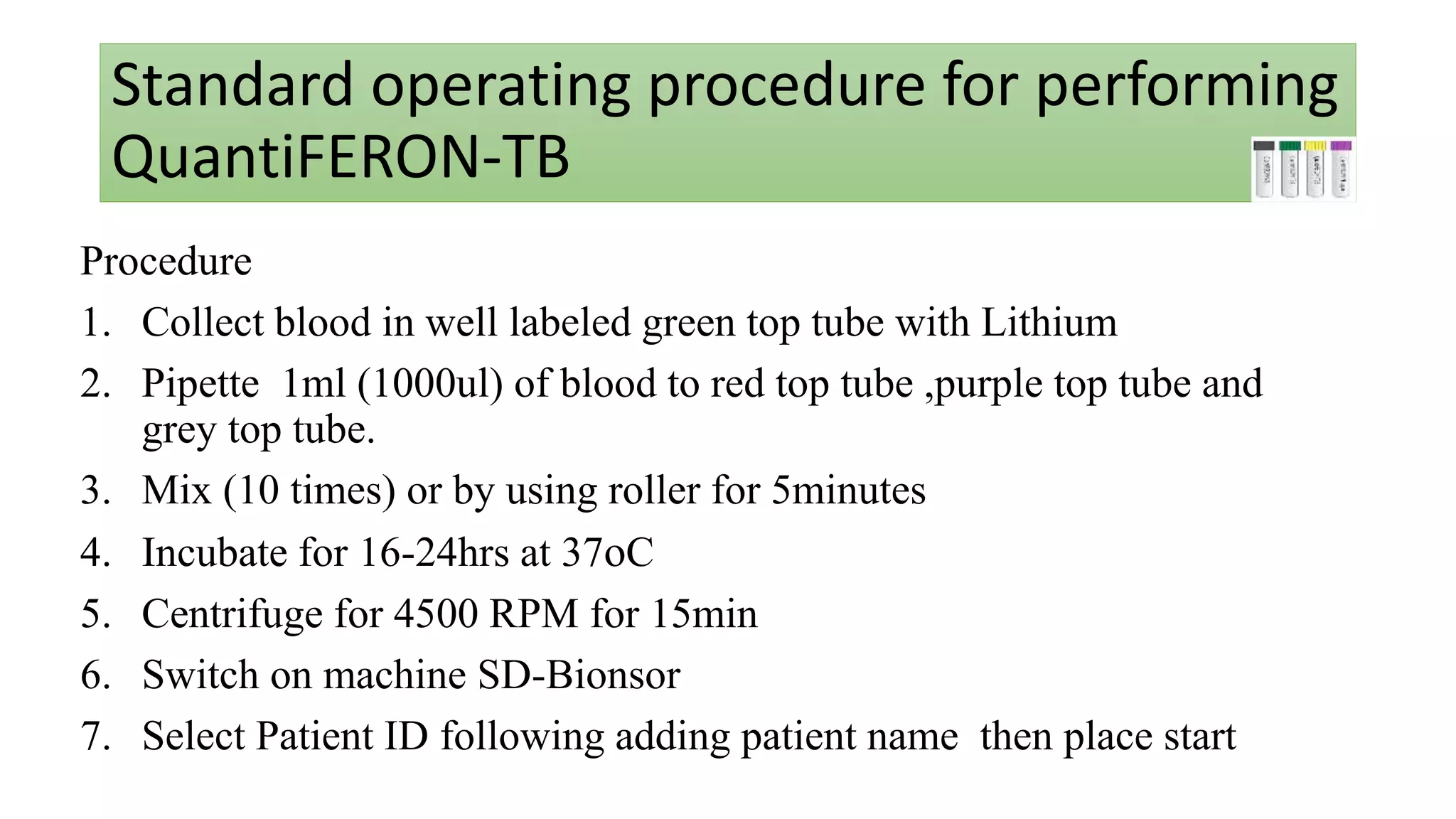
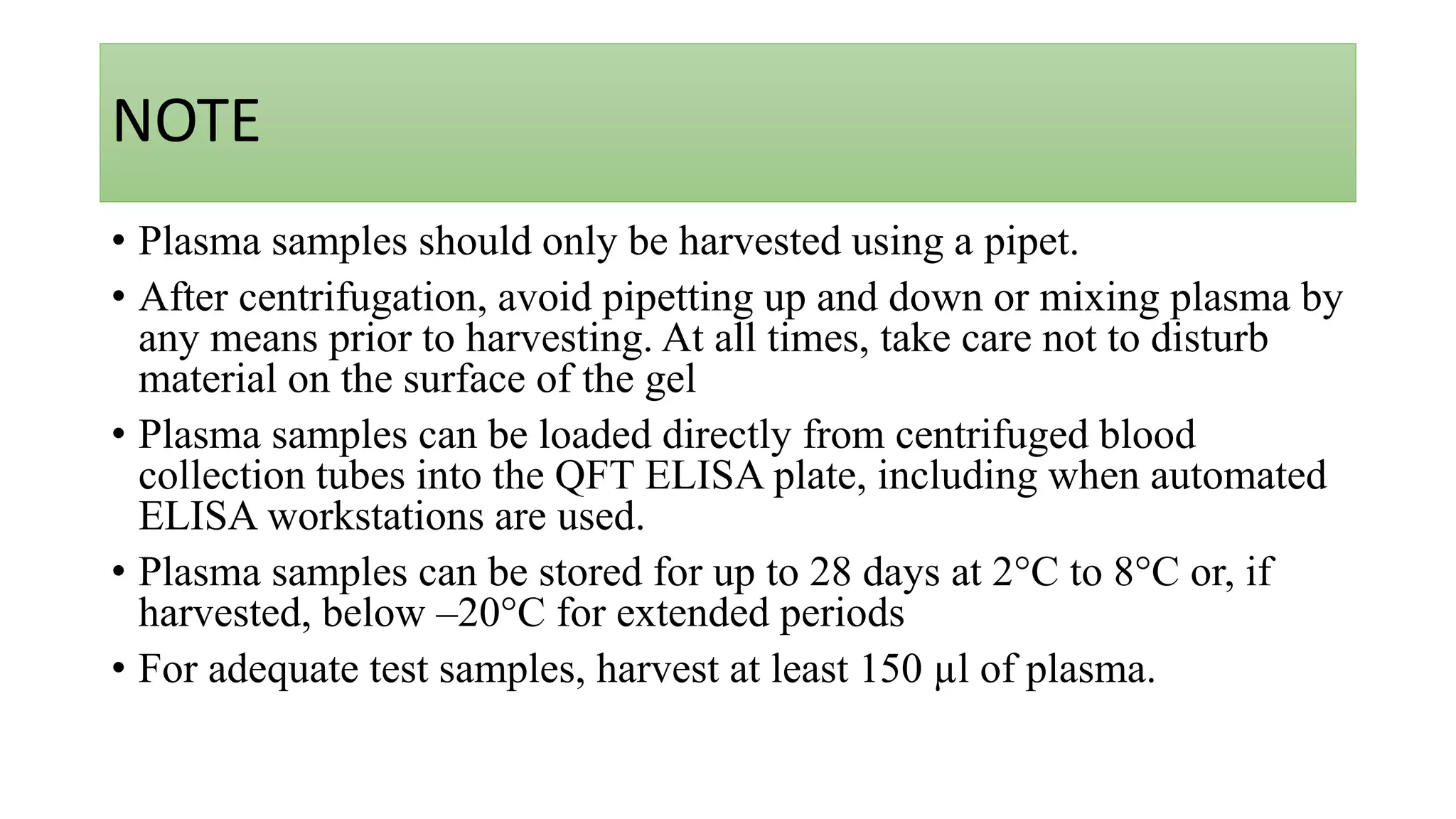
Quantiferon-TB Gold (QFT) is a blood test that detects tuberculosis by measuring immune responses to specific TB antigens. It is a more reliable alternative to traditional skin tests, requiring only one patient visit and being unaffected by BCG vaccination. QFT results provide critical information for diagnosing TB infection but cannot differentiate between active disease and latent infection.