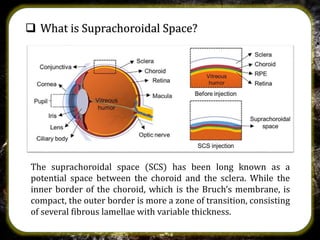
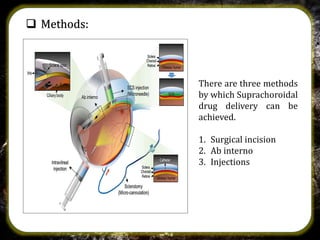
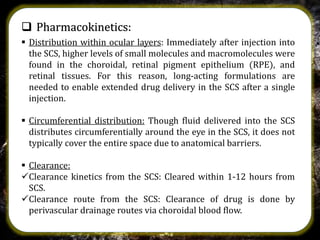
The suprachoroidal drug delivery system is a novel approach that utilizes the suprachoroidal space for ophthalmic therapeutic agent administration, offering high bioavailability and targeted drug delivery. Compared to traditional routes like eye drops and intravitreal injections, it minimizes ocular side effects and may enhance safety and efficacy. Research is ongoing, with microneedle injection methods entering clinical trials and further exploration of its applications for treating posterior segment diseases of the eye.