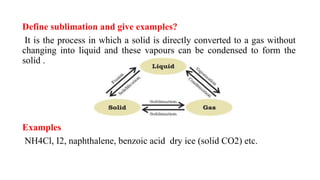
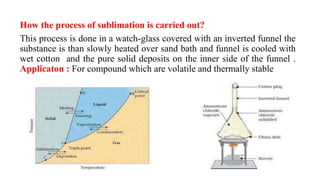
Sublimation is the direct change of a solid substance into a gas without passing through the liquid state. Examples of substances that sublime include NH4Cl, I2, naphthalene, and benzoic acid. The pure solid obtained after sublimation is called the sublimate, while the original solid undergoing sublimation is the sublimand. Sublimation is carried out by slowly heating the sublimand over a sand bath under an inverted funnel that is cooled to cause the gaseous sublimate to condense on the inner side of the funnel. Sublimation is used to purify volatile thermally stable compounds and separate them from non-volatile substances.