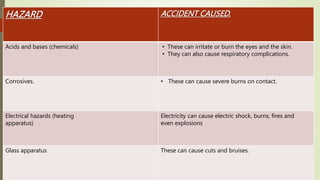
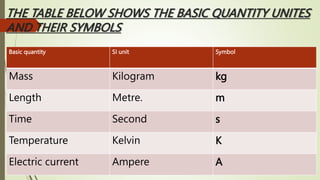
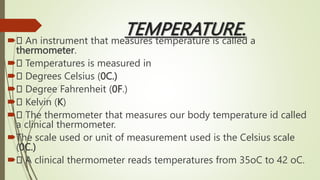
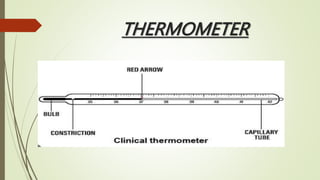
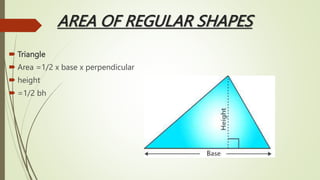
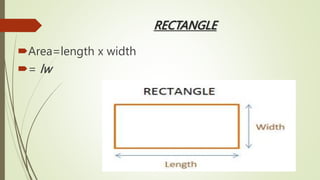
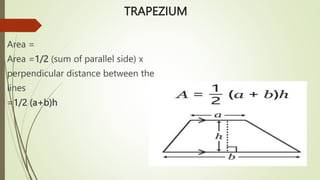
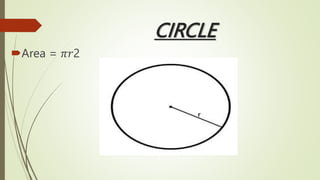
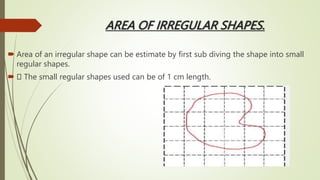
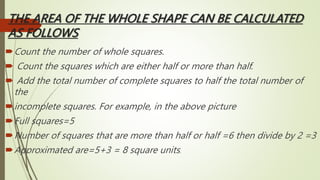
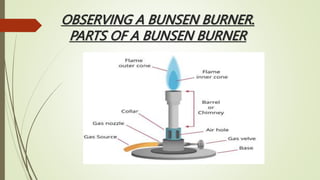
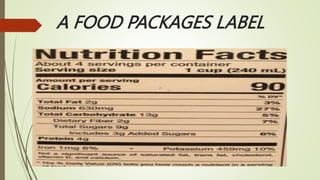
The document provides information about integrated science and scientific investigations. It defines integrated science as combining the five basic natural sciences of physics, chemistry, biology, earth science and astronomy. It discusses the importance of integrated science in daily life through applications in transportation, medicine, agriculture and communication. The document also covers laboratory safety, including common hazards, accidents, first aid measures, hazard symbols and safety regulations in the laboratory.