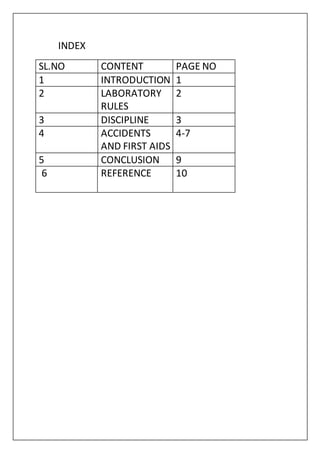
This document discusses laboratory rules, discipline, and first aid in physical science education. It outlines 10 rules for the laboratory, including only allowing students to perform assigned experiments under teacher supervision and properly storing chemicals and equipment. Discipline in the laboratory requires strict guidelines to maintain safety. The document also describes various common accidents that may occur, such as poisoning, inhalation of gases, fires, eye injuries, burns, and electric shock, and provides first aid recommendations for each, emphasizing calling a doctor for more serious injuries. Maintaining safety rules and providing first aid training is important for ensuring a safe learning environment in the physical science laboratory.