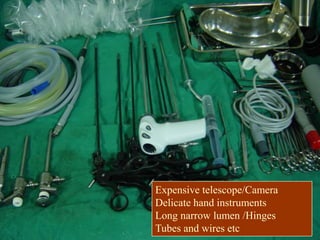
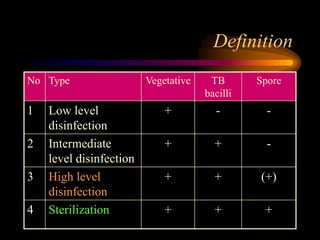
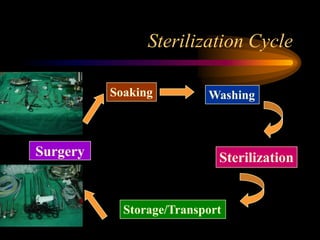
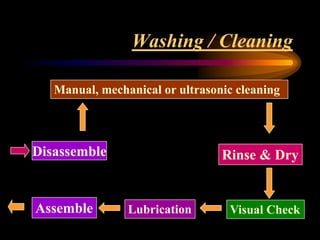
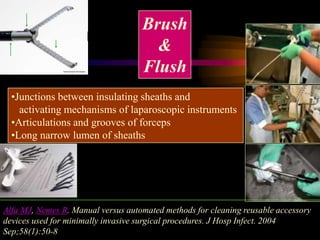
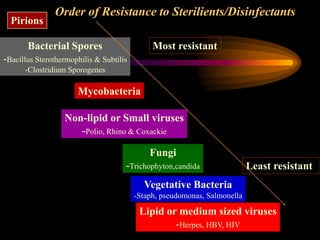
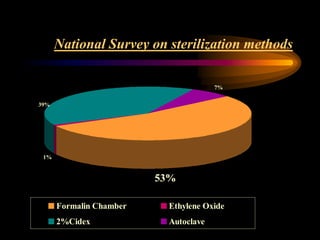
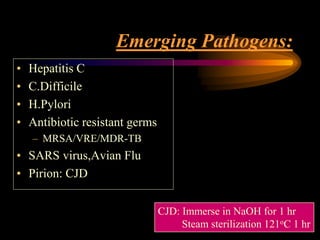
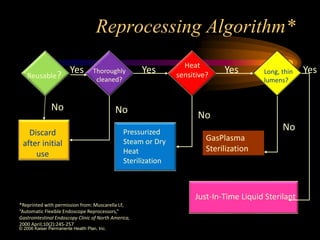
Three great pioneers in sterilization and disinfection were Ignaz Semmelweis, Louis Pasteur, and Joseph Lister. Laparoscopic equipment is considered "critical" due to entering sterile body tissues, requiring sterilization or high-level disinfection. Proper cleaning and sterilization/disinfection of laparoscopic equipment is essential to prevent surgical site infections according to guidelines. Steam sterilization, ethylene oxide, and high-level disinfection with chemicals like glutaraldehyde or hydrogen peroxide are commonly used methods to sterilize or disinfect laparoscopic equipment.