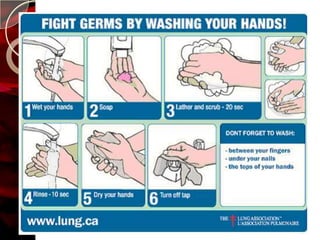
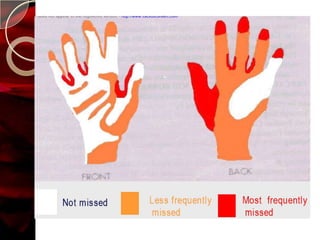
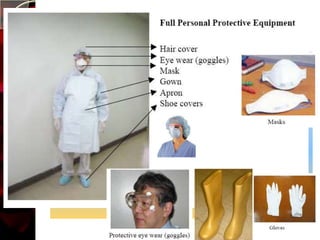
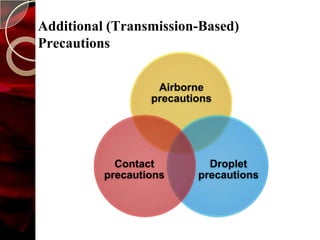
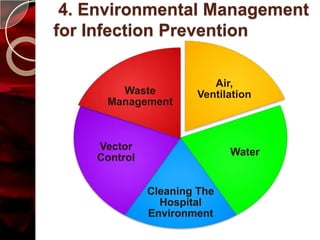
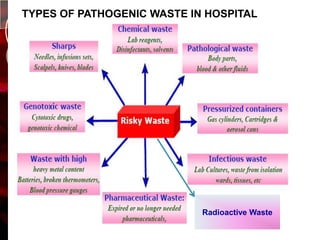
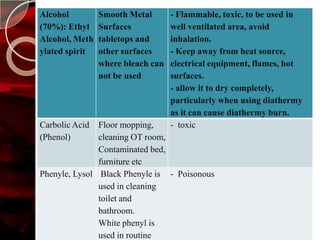
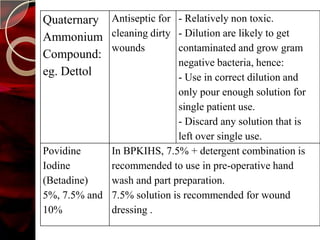
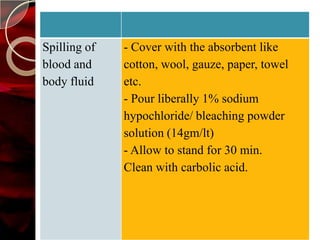
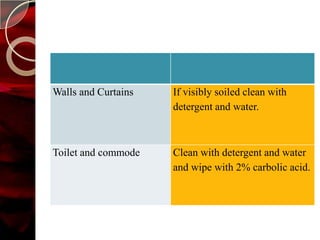
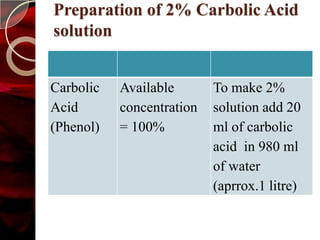
The document discusses infection control practices in hospitals, including how infections spread, standard and transmission-based precautions, environmental management, and methods for decontamination, sterilization, and disinfection. It provides details on the various disinfectants used in the hospital and guidelines for cleaning different equipment and environmental surfaces. The history and importance of infection control is also reviewed.





![Background
Infection rate in developing countries was 15.5 per 100
patients, compared to “.1 [per 100 patients] in Europe
and in the U.S., 4.5- BBC
ICU infection - developing countries: 47.9 per 1,000
patient, compared to 13.6 in the U.S.
In countries like India and Nepal, hospital infection data
not reliably estimated.
Surgical infection at BPKIHS -1339 (7.3%) among
18325 total surgeries.
Estimates vary from 10 to 30%, the least being about
3% in the best of hospitals
Wound sepsis alone affects 20% of post-operative cases](https://image.slidesharecdn.com/infectioncontrol-121016015356-phpapp01/85/Infection-control-6-320.jpg)