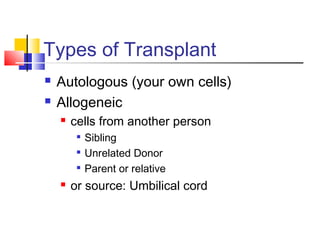
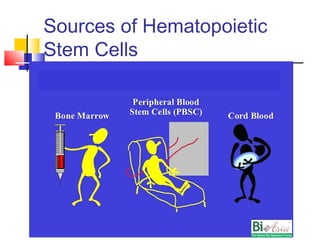
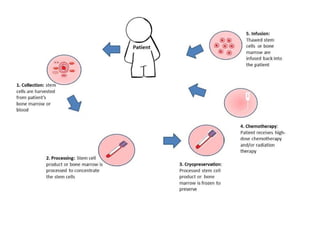
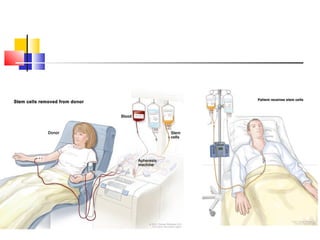
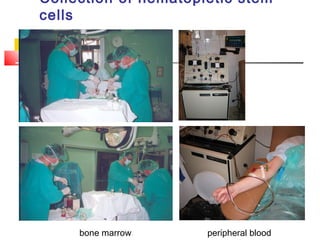
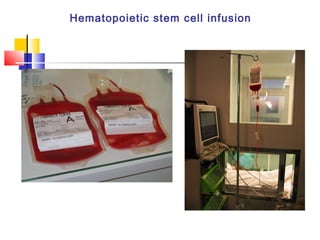
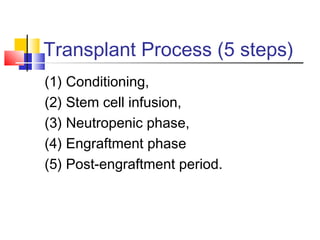
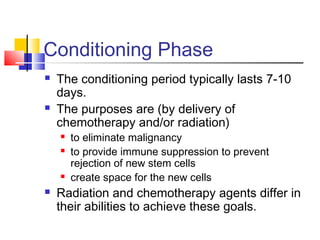
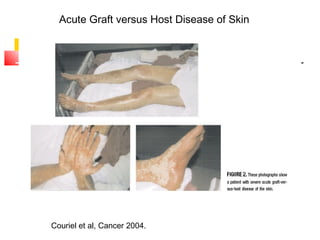
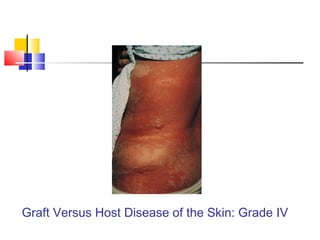
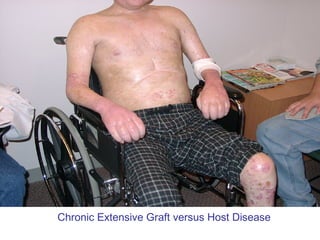
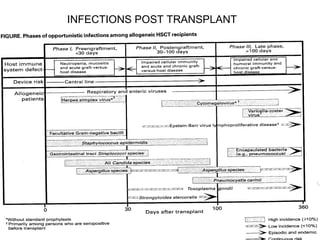
Hematopoietic stem cell transplantation involves transplanting stem cells from bone marrow, peripheral blood, or umbilical cord blood to a recipient. There are two main types: autologous transplants using the patient's own stem cells and allogeneic transplants using donor stem cells. Allogeneic transplants from related donors have the best outcomes. The transplant process involves conditioning with chemotherapy and/or radiation, stem cell infusion, a neutropenic phase making the patient susceptible to infection, engraftment as the donor cells establish, and a post-engraftment period monitoring for complications like graft-versus-host disease.