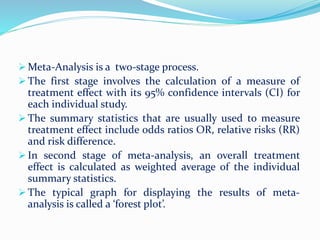
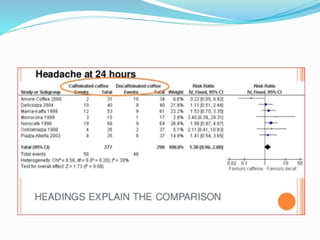
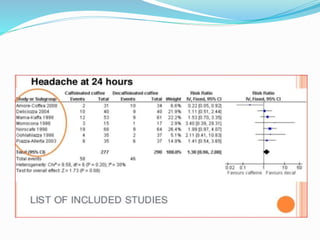
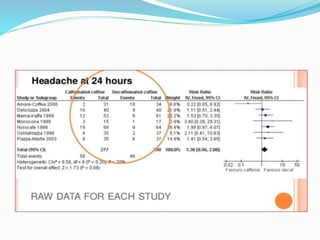
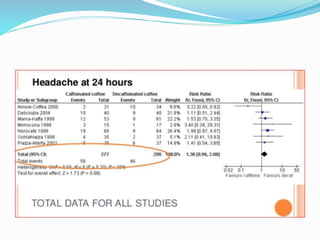
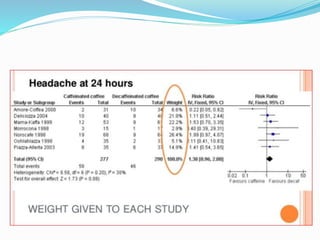
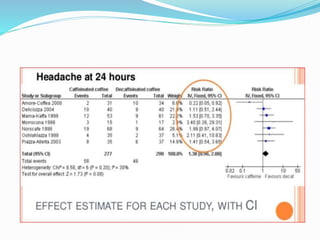
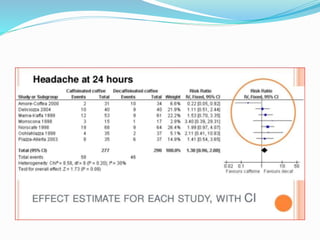
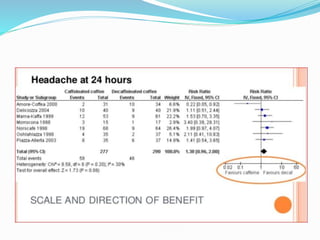
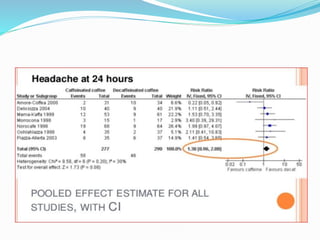
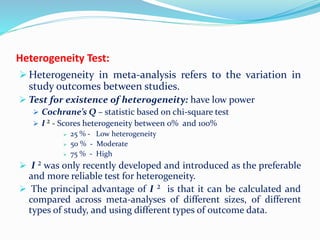
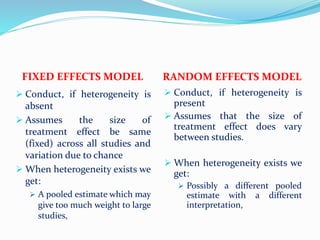
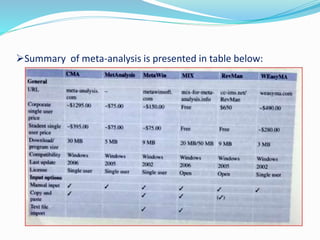
This document provides an overview of statistics used in meta-analysis. It discusses key concepts like odds ratios, relative risk, confidence intervals, heterogeneity, and fixed and random effects models. It also summarizes different types of meta-analyses including realist reviews, meta-narrative reviews, and network meta-analyses. Software for performing meta-analyses and potential pitfalls in systematic reviews are also briefly covered.