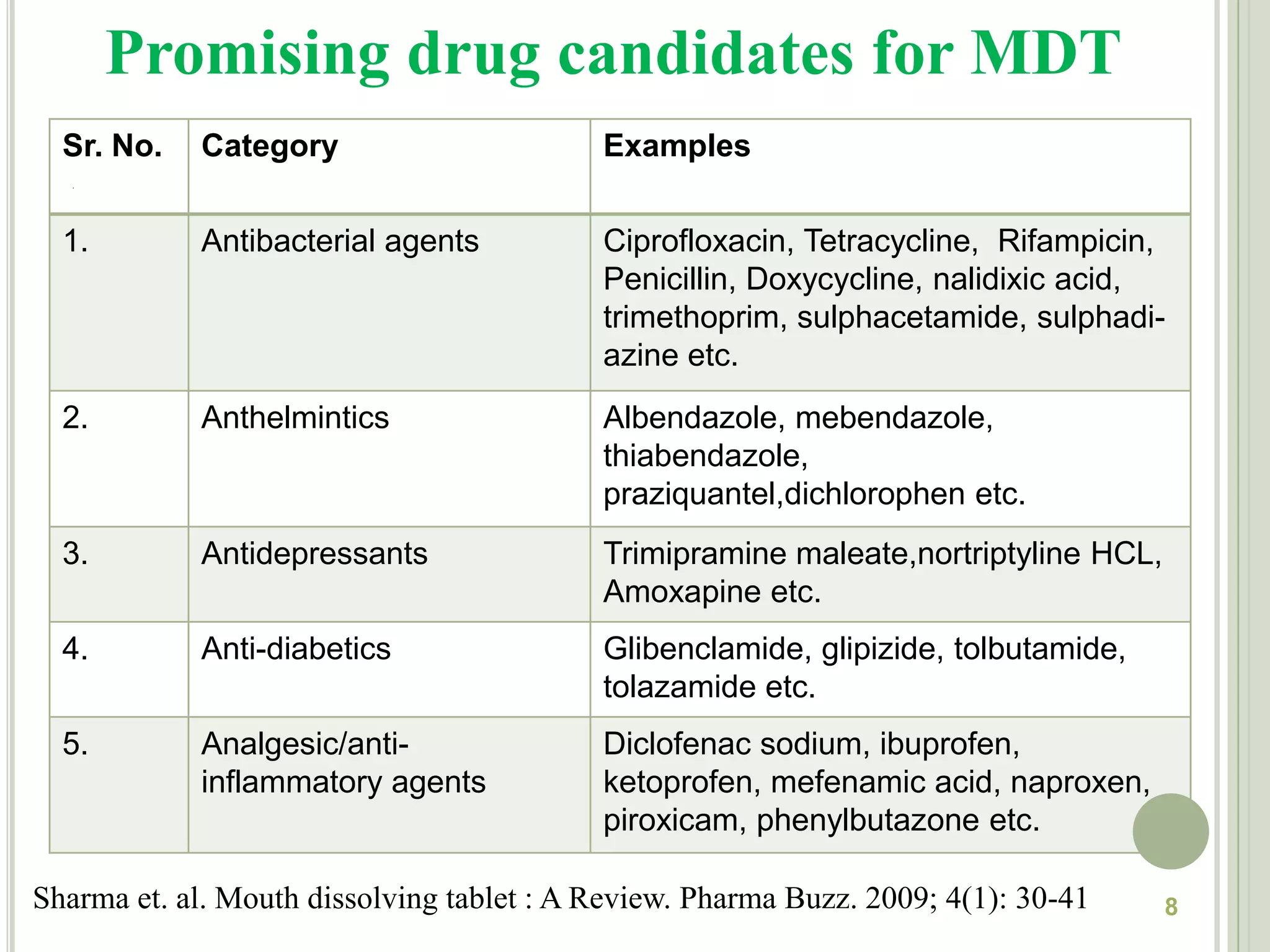
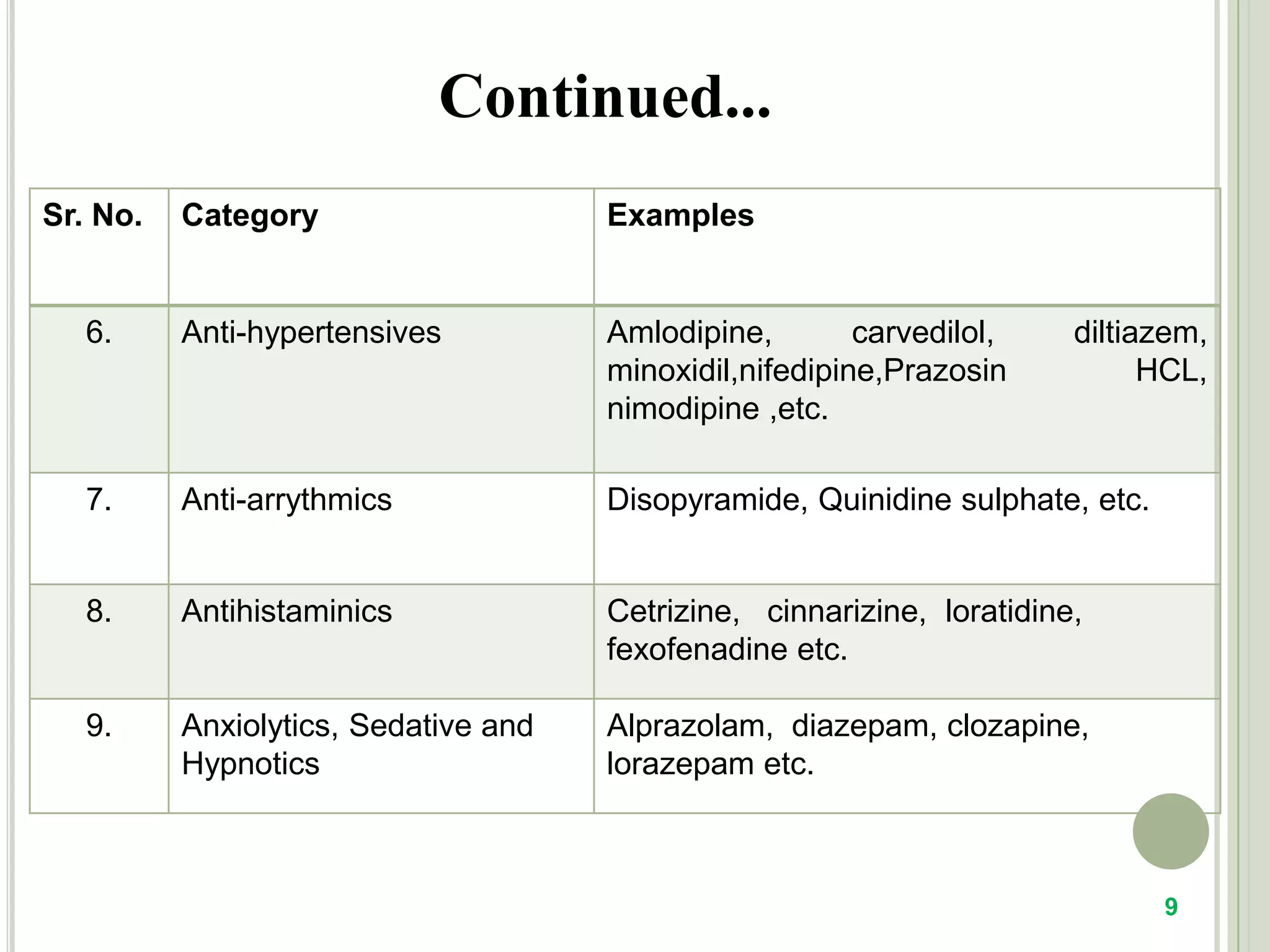
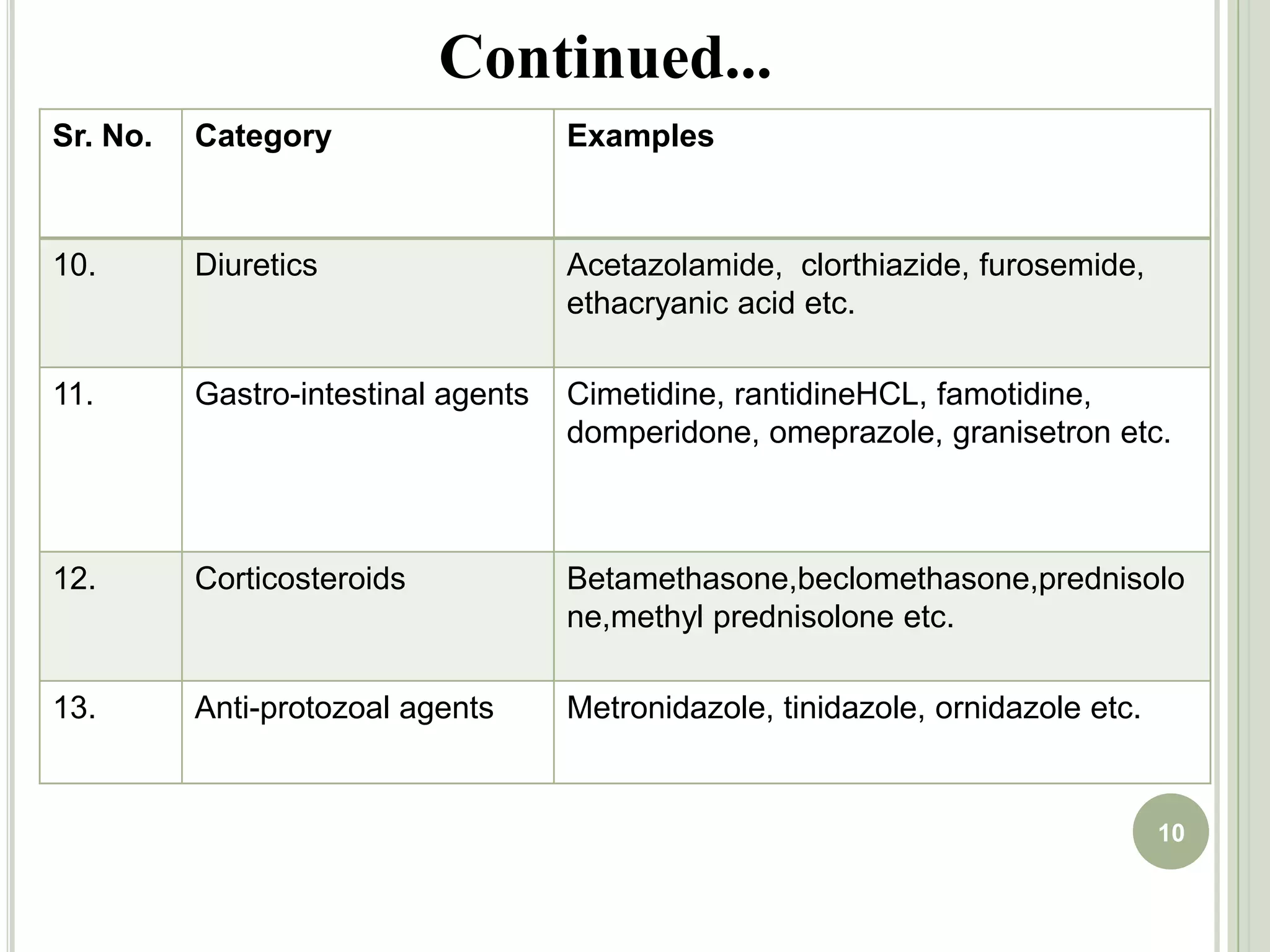
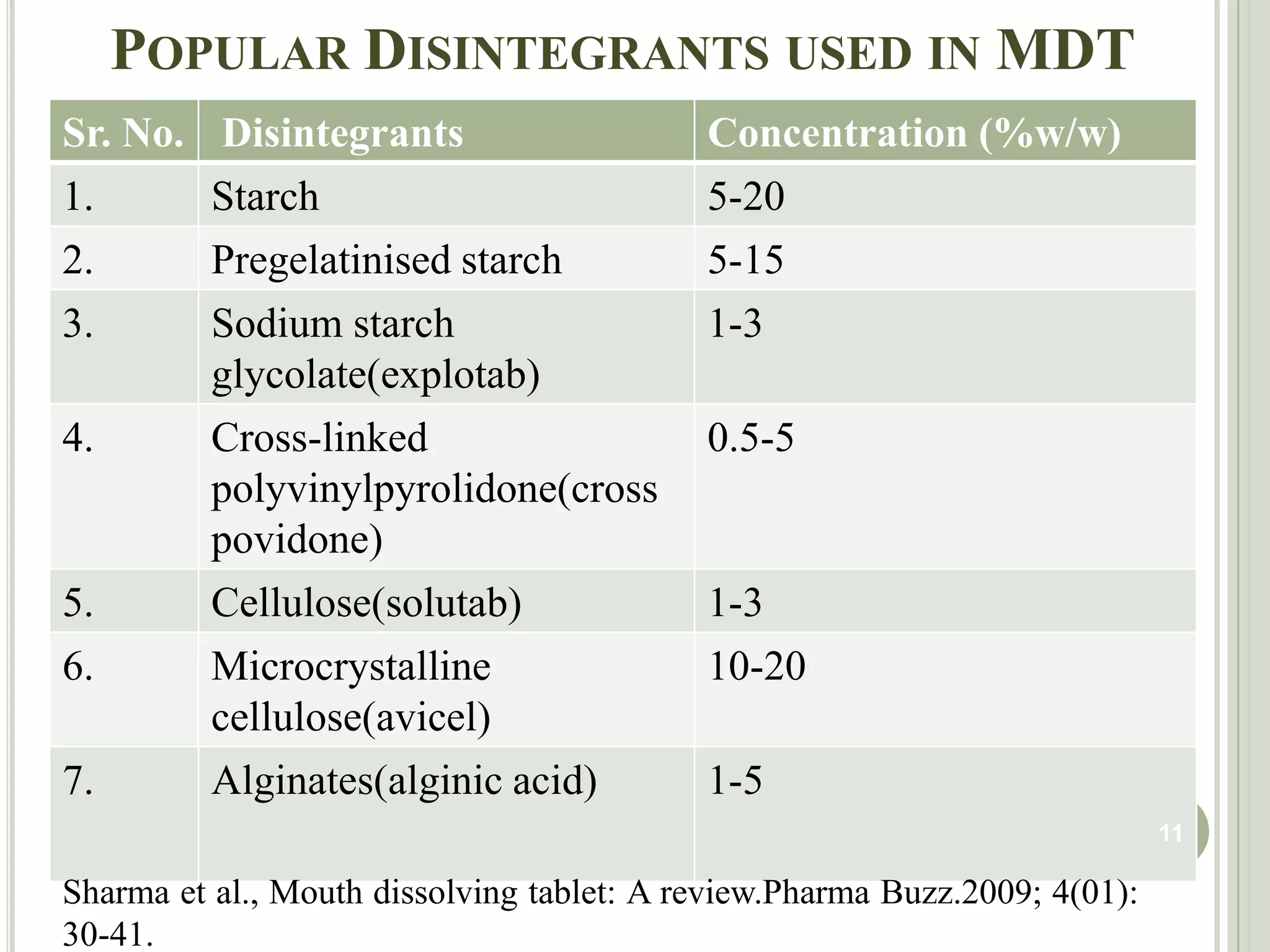
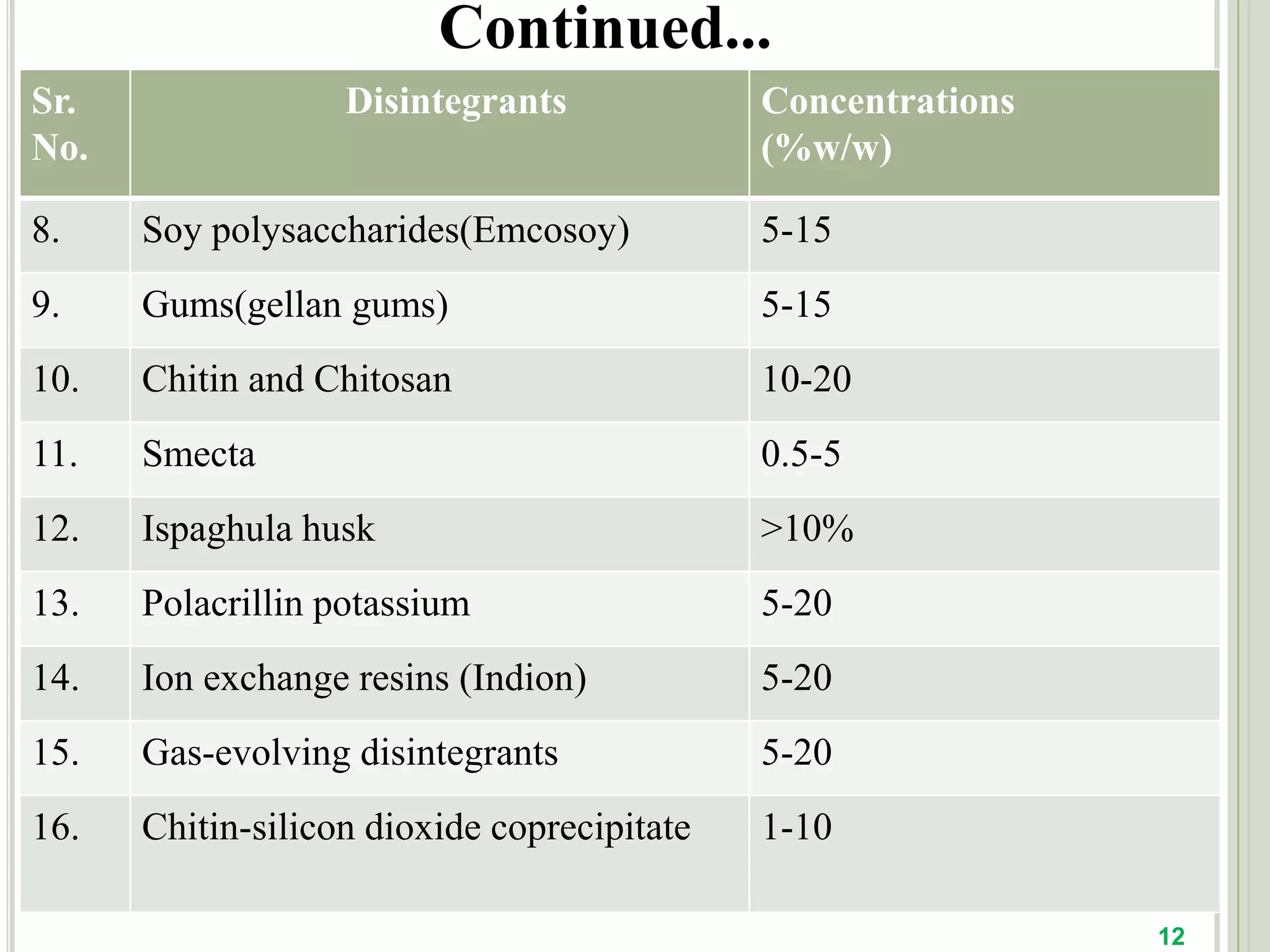
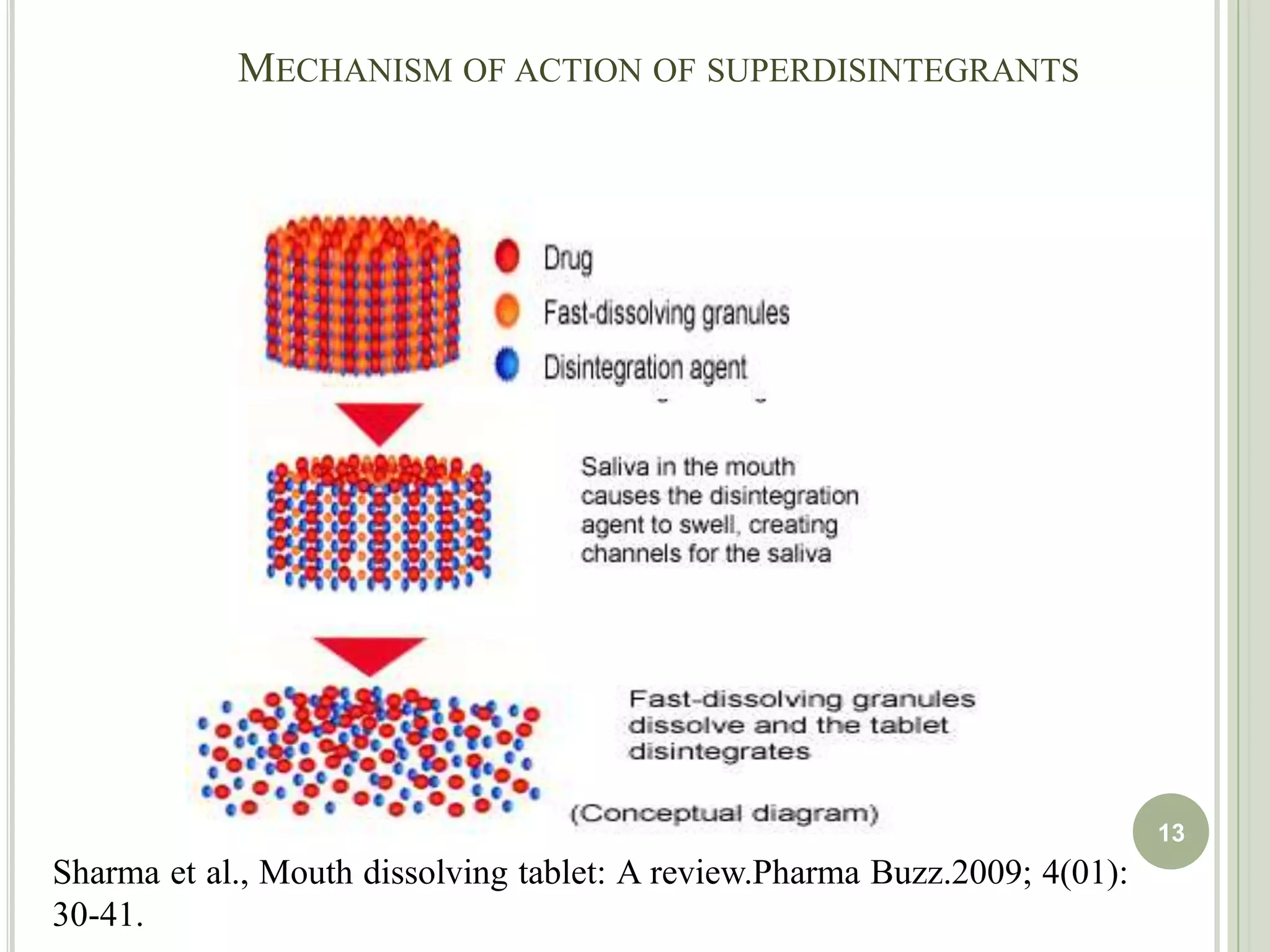
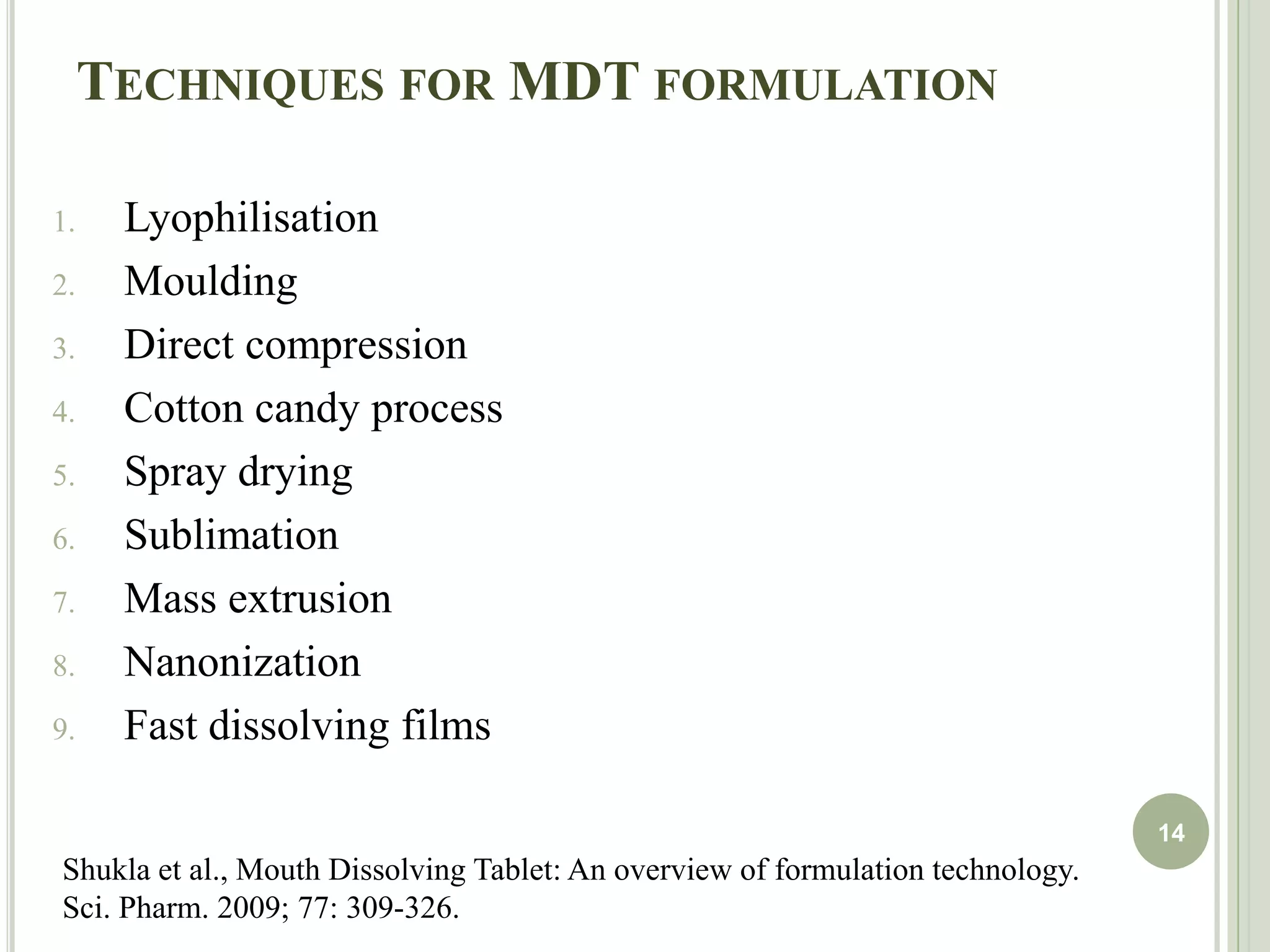
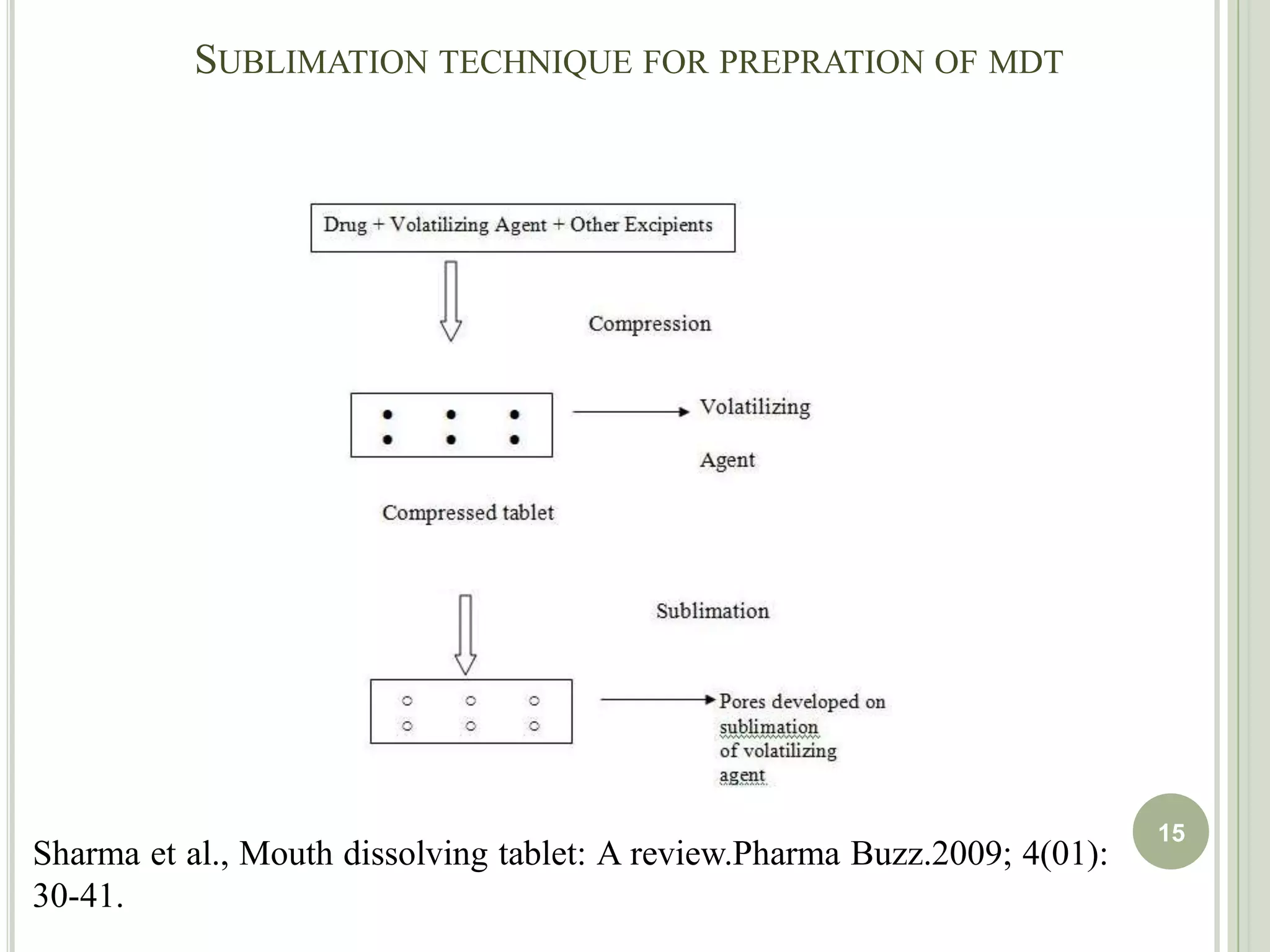
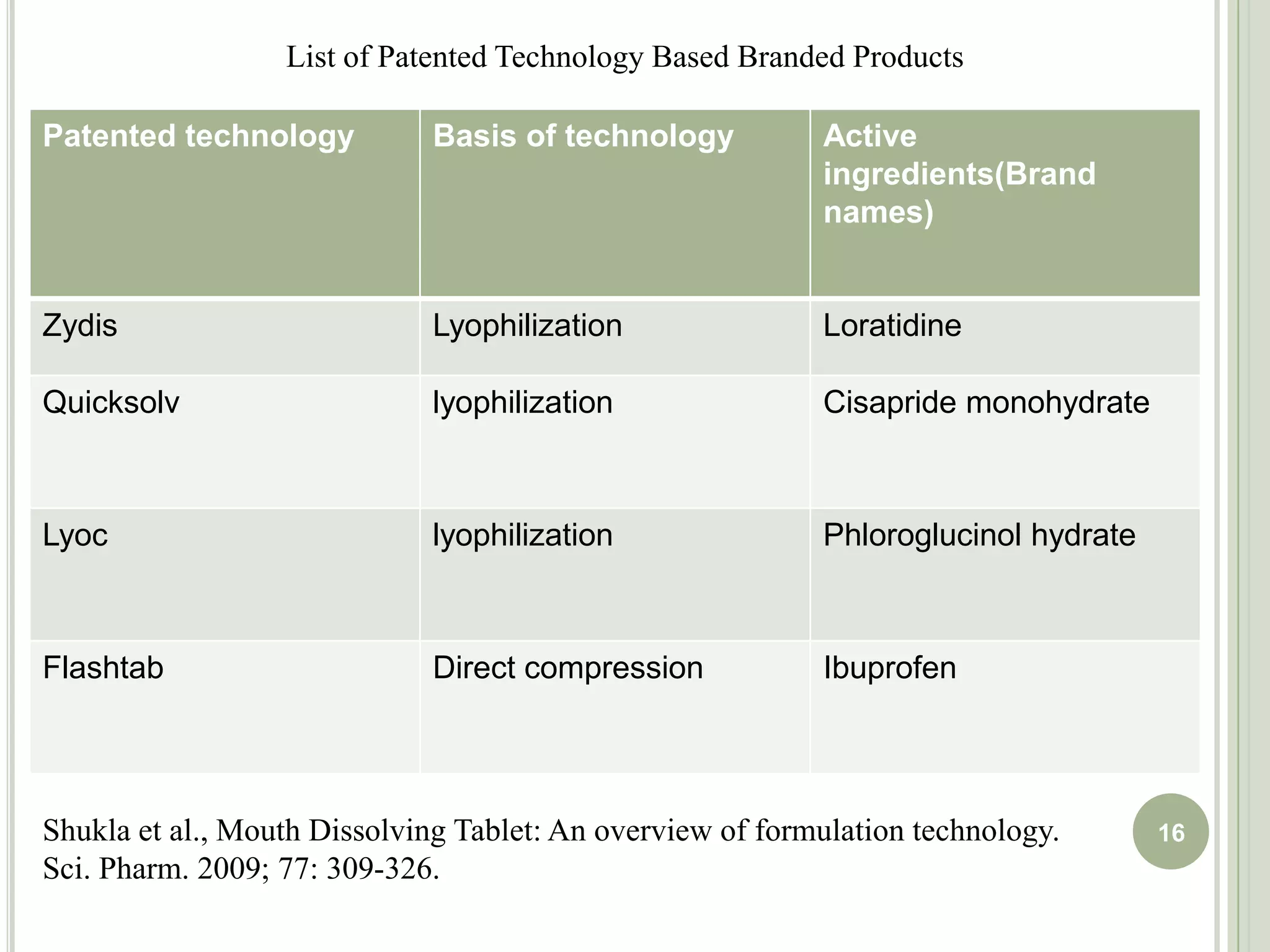
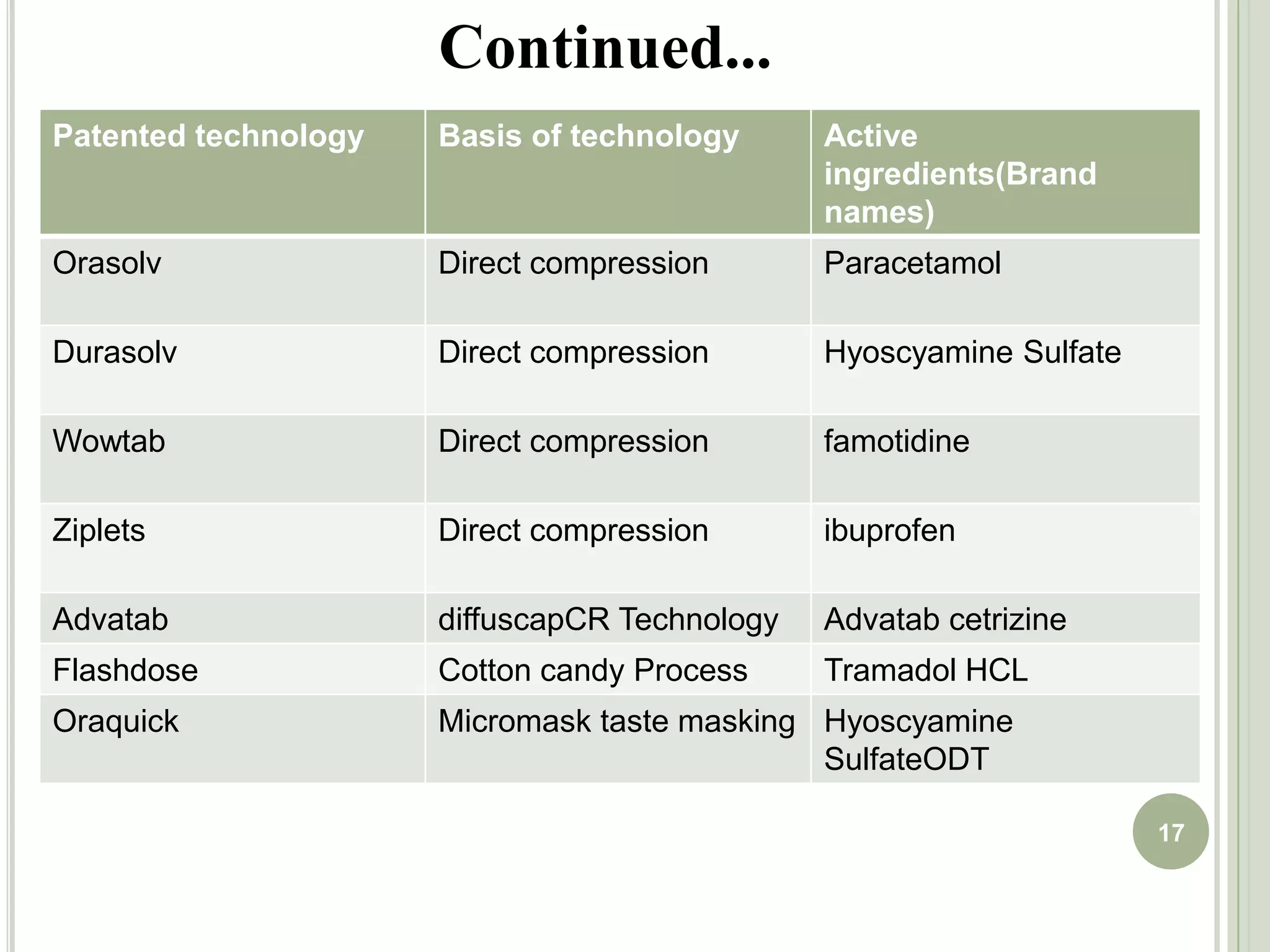
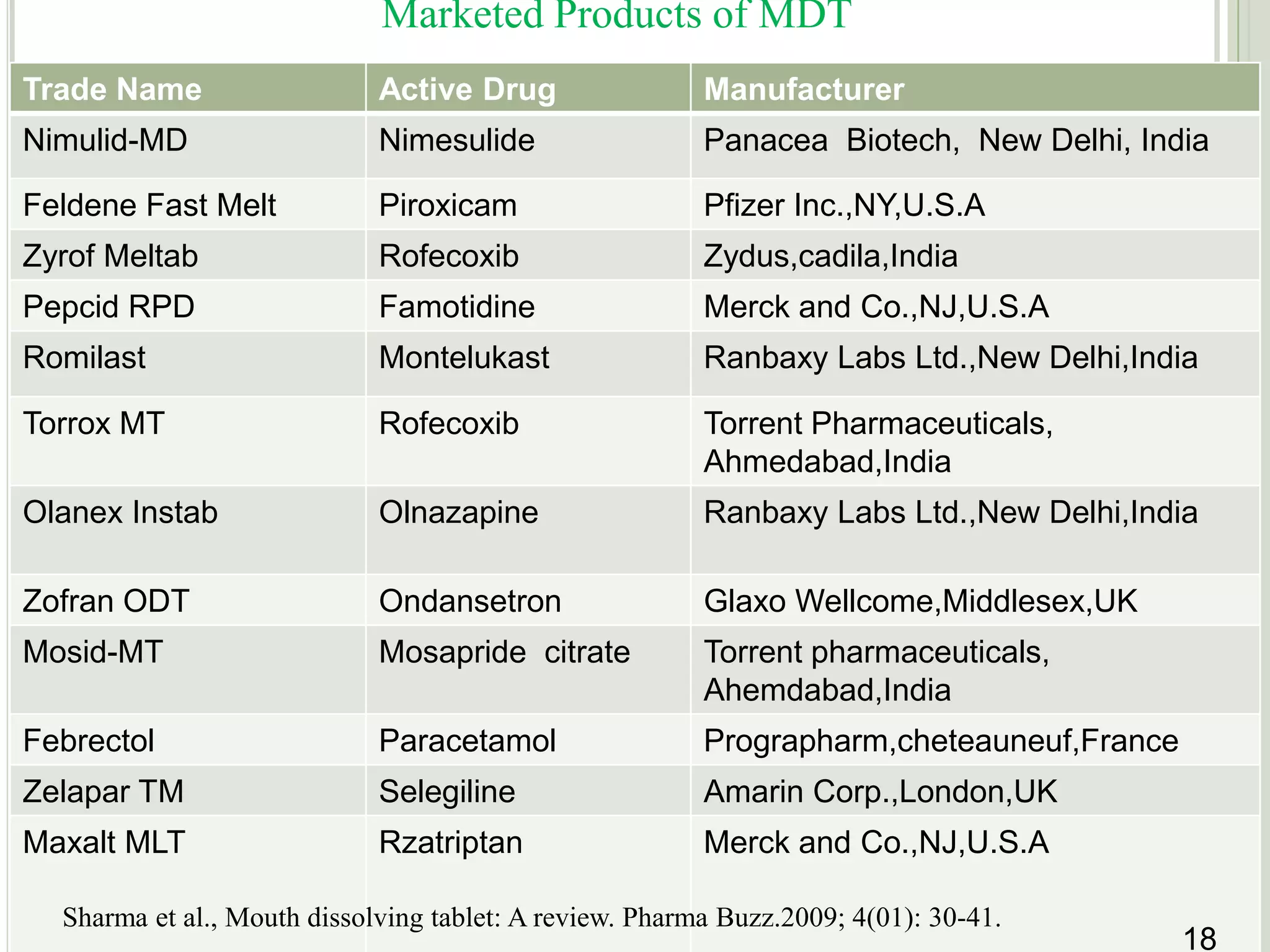
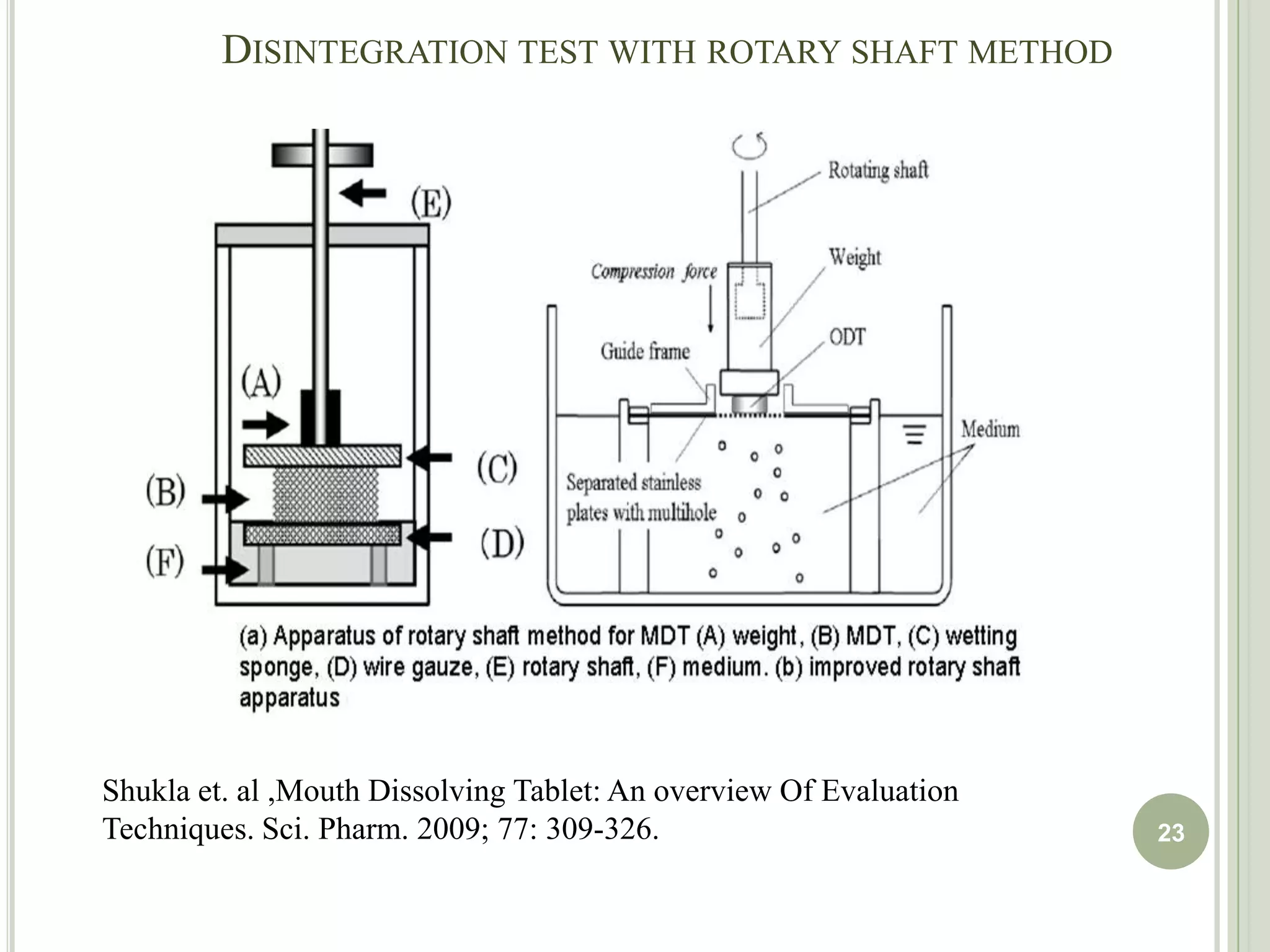
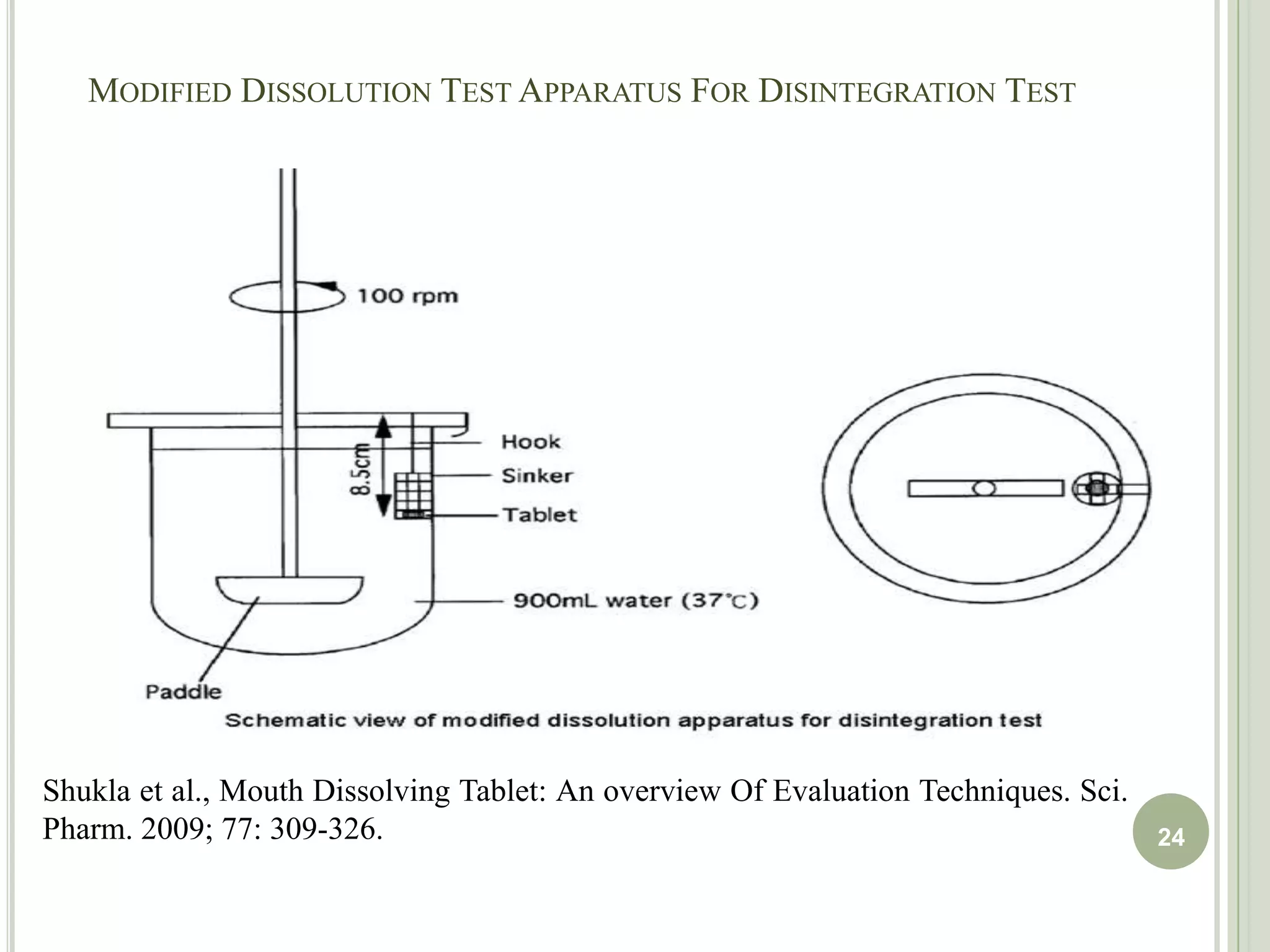
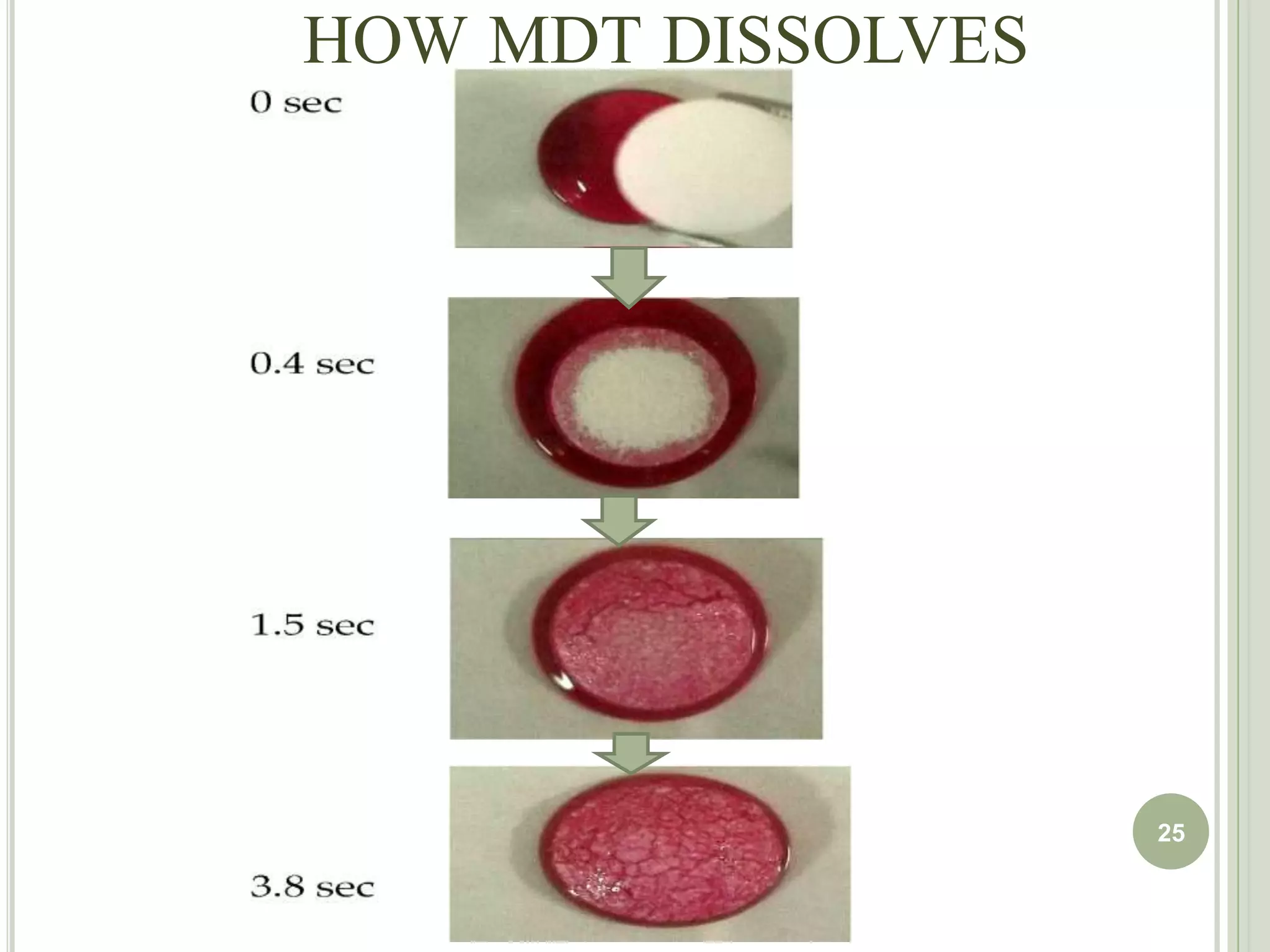
This document provides an overview of mouth dissolving tablets (MDT), including promising drug candidates, popular disintegrants used, formulation techniques, marketed products, and evaluation methods. MDTs are designed to dissolve rapidly in the mouth within 3 minutes before swallowing for improved patient compliance. Common techniques for MDT formulation include lyophilization, moulding, direct compression, and sublimation. Evaluation of MDTs involves testing of granules, tablets, and dissolution properties. Future research aims to identify suitable drug candidates and excipients while improving dissolution times and patient acceptability.