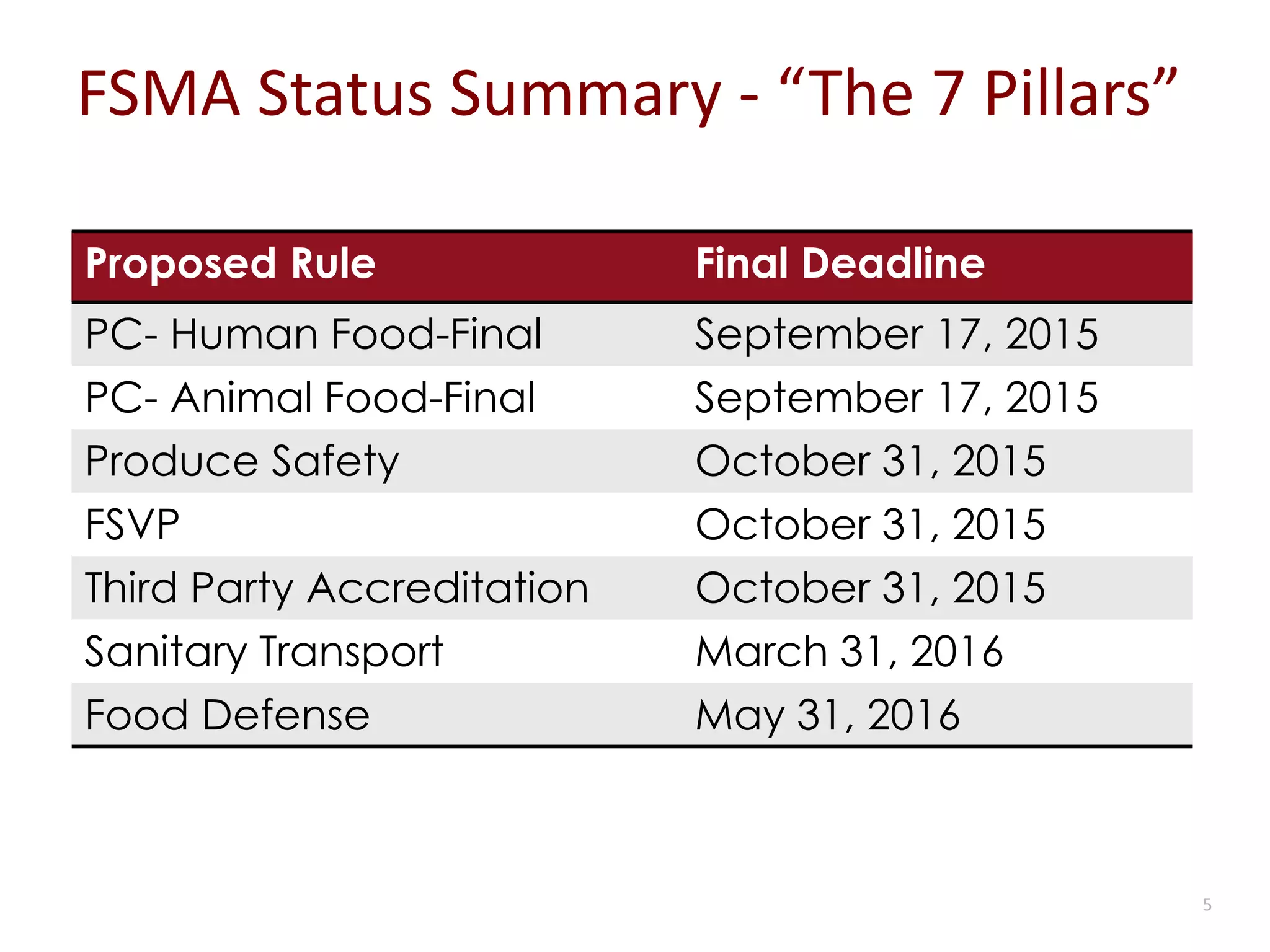
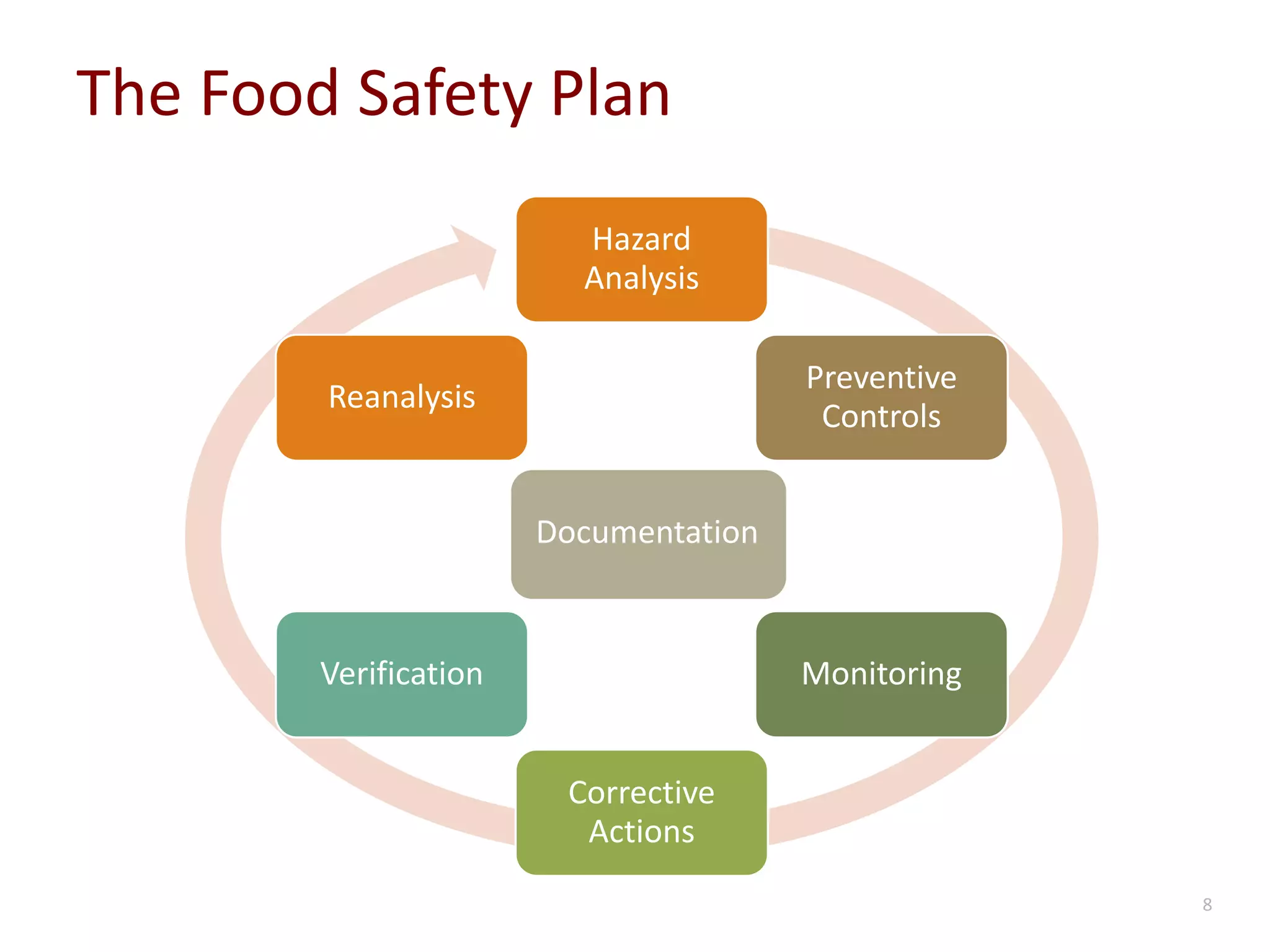
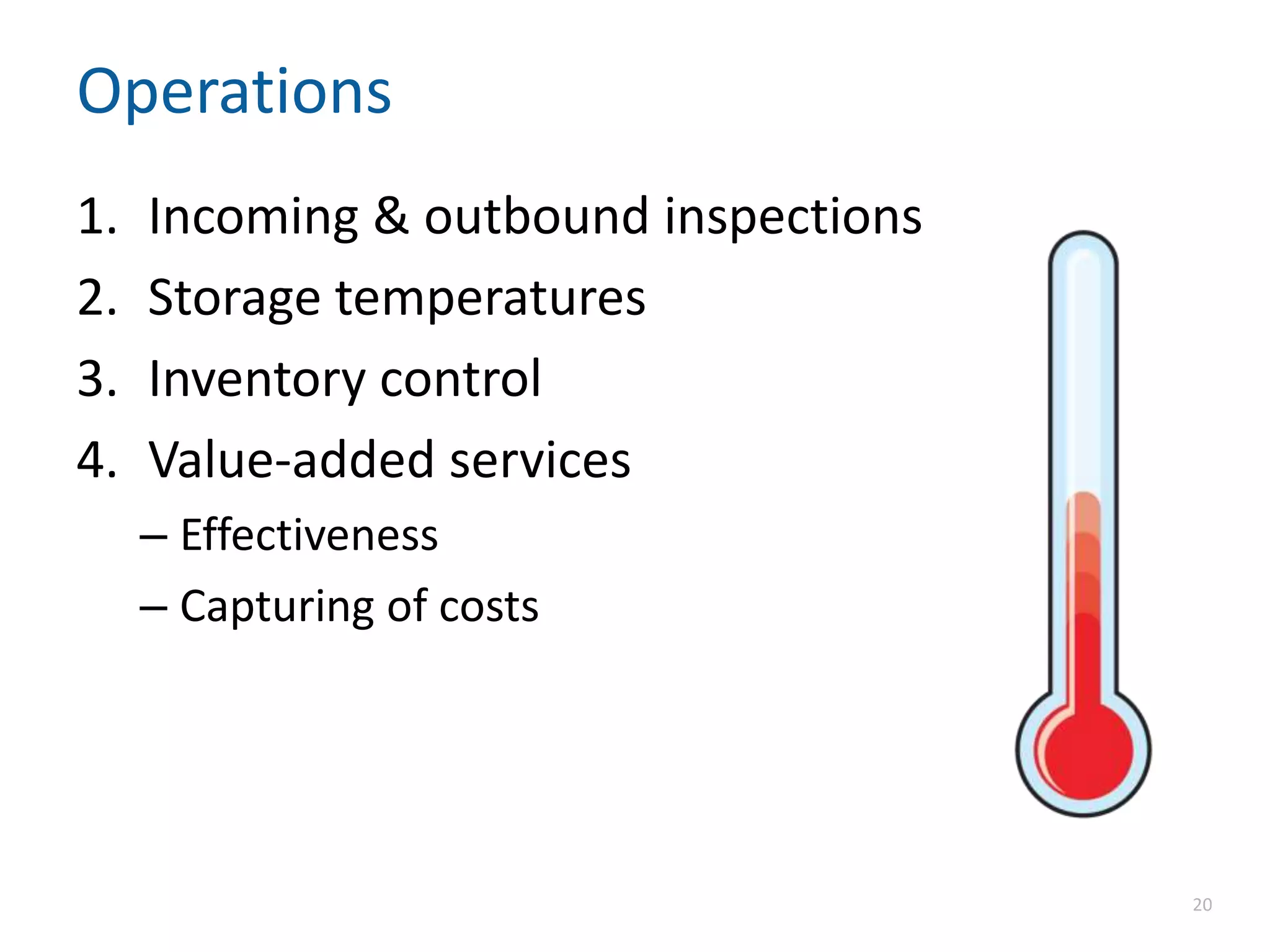
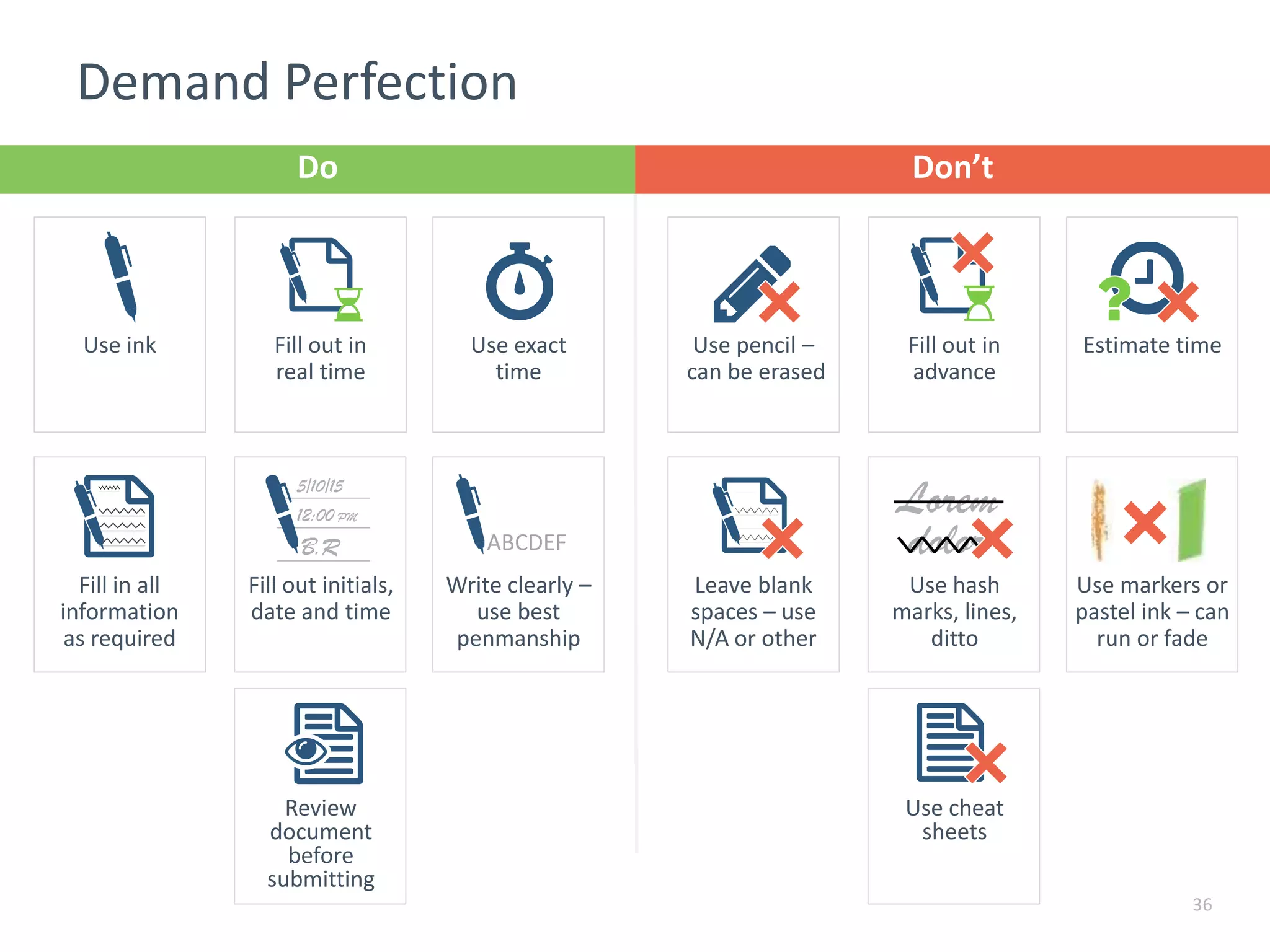
The document discusses the importance of proper recordkeeping and documentation in food safety to reduce risk and liabilities, outlining compliance requirements under the FSMA. It emphasizes the need for a written food safety plan, detailed recordkeeping for hazard analyses, preventive controls, and supplier verification, with a focus on legal considerations and audit requirements. Common pitfalls in documentation practices are identified, alongside a call for organizational discipline and the importance of consistency in recordkeeping.