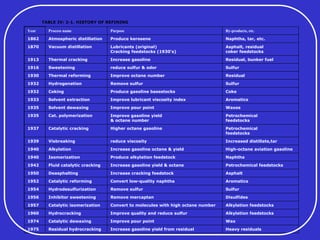
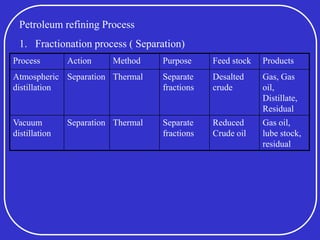
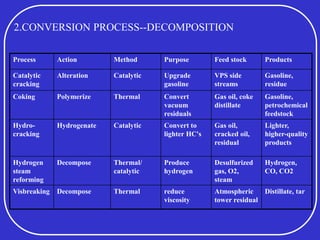
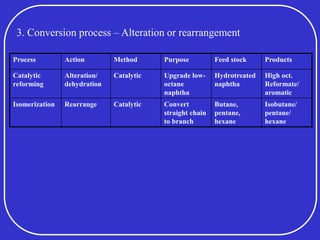
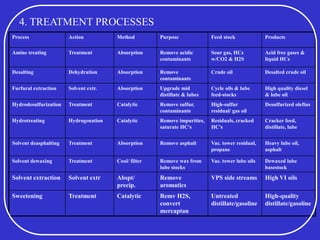
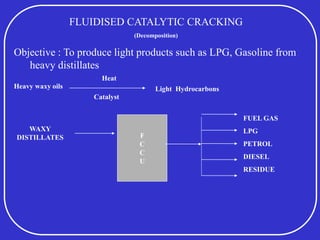
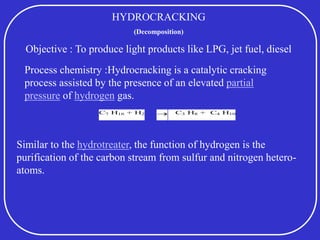
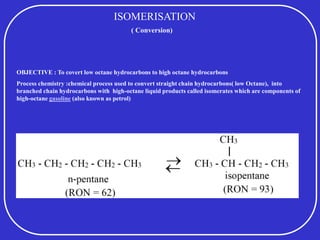
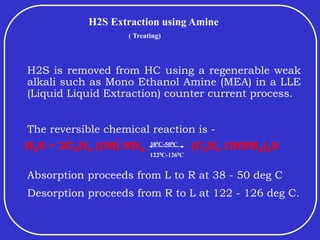
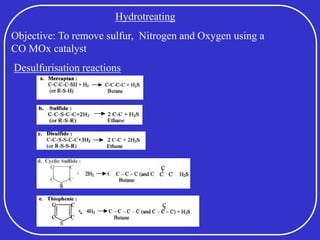
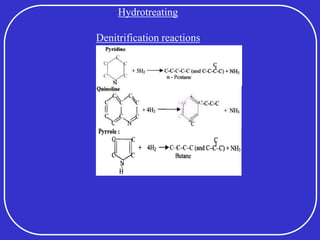
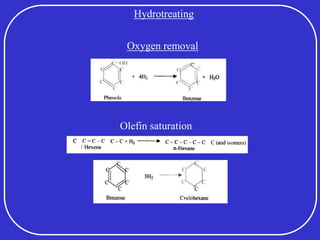
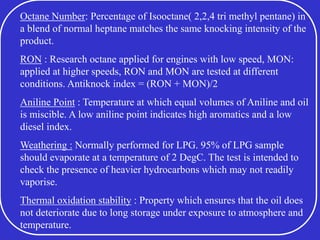
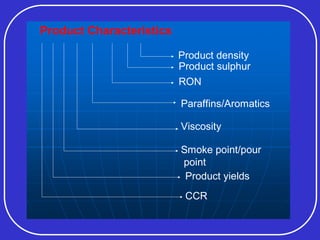
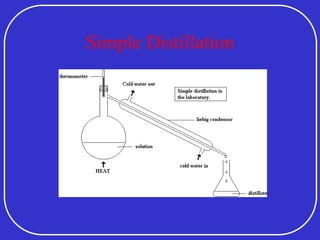
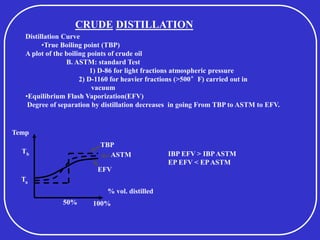
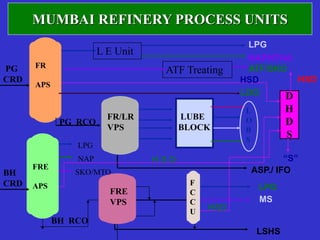
Crude oil is a complex mixture formed from the decay of living matter, predominantly consisting of hydrocarbons with varying compositions and properties. It can be classified based on API gravity into light, medium, and heavy crude, and undergoes various refining processes to separate and enhance its components. Key characteristics of crude oil include viscosity, cloud point, pour point, flash point, and sulfur content, which are essential for processing and determining product quality.












![Crudes
High Low
Sulphur Sulphur
Lower/Upper
Zakum, Murban
(UAE)
Dubai Kuwait
Bonny Lt.
(Nigeria)
Essider
(Libya)
Brent
Blend
(N Sea)
35% 65%
[‘02-’03] [‘02-’03]
BH/Ravva
(Indg)](https://image.slidesharecdn.com/refinerychemistry1-240630181253-fa4e3216/85/Refinery-chemistry-for-non-technical-backgorund-22-320.jpg)
![Gross Refinery Margin [GRM]
• To access the profitability of the crude
GRM = Product Value –
Crude Cost –
Variable Costs.
Variable Costs
• They are the costs of various chemicals and utilities
used while processing the crude.
• Depend on the grade of the crude being
processed.](https://image.slidesharecdn.com/refinerychemistry1-240630181253-fa4e3216/85/Refinery-chemistry-for-non-technical-backgorund-24-320.jpg)