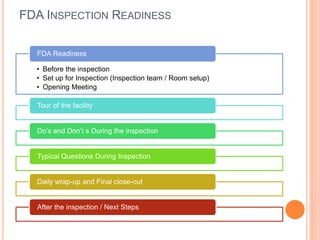
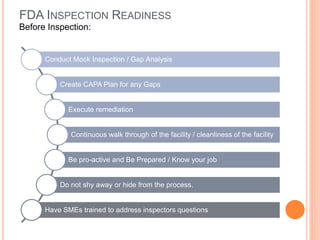
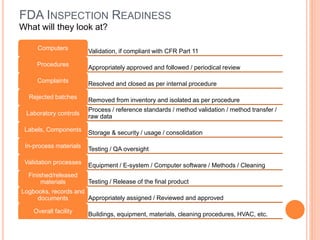
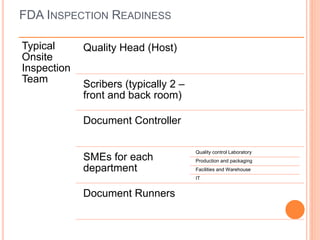
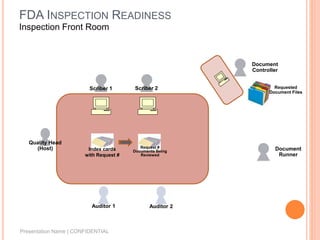
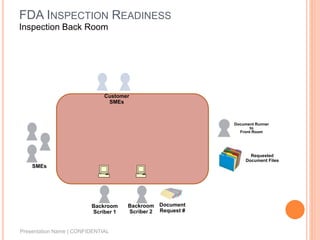
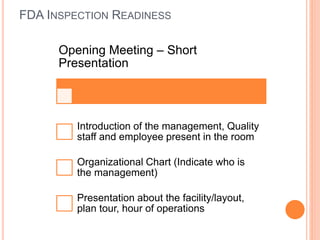
The document provides guidance on preparing for and managing an FDA inspection. It outlines steps to take before, during, and after the inspection including conducting a mock inspection, setting up an inspection team, and documenting any deficiencies found. It also provides dos and don'ts for interacting with inspectors such as being prepared, transparent, and avoiding arguments.