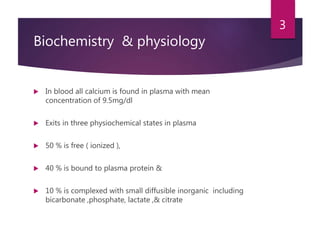
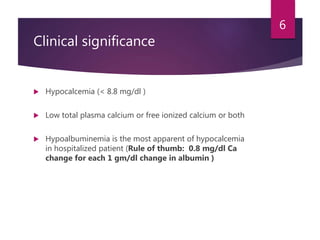
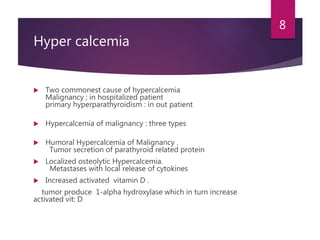
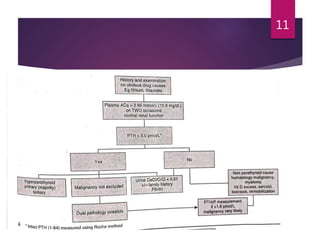
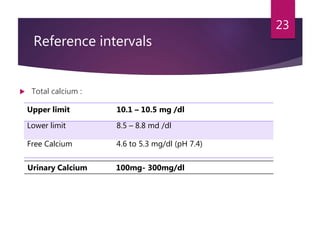
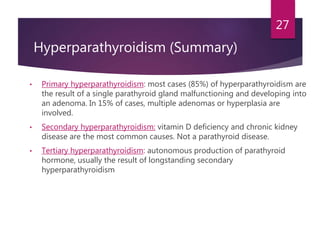
This patient is most likely suffering from primary hyperparathyroidism. Two reasons support this diagnosis: 1) fluctuating calcium levels are typical of primary hyperparathyroidism and 2) a higher calcium level should be accompanied by a low PTH, but this patient has a high PTH with higher calcium levels. The commonest cause of primary hyperparathyroidism is a single adenoma of the parathyroid gland, present in around 85% of cases.