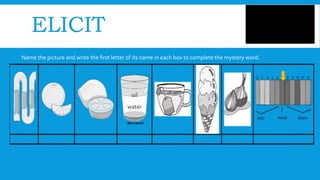
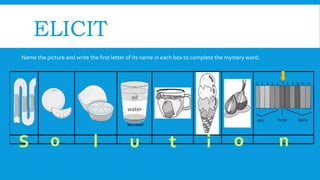
Here are some factors that affect solubility:
1. Temperature - In general, the higher the temperature, the more soluble a substance becomes. As temperature increases, the kinetic energy of the particles increases, allowing them to overcome attractive forces and dissolve more easily.
2. Pressure - For gases dissolving in liquids, increased pressure favors dissolution by compressing the gas and forcing it into the liquid.
3. Nature of the solute - Polar substances like salts, sugars, and acids are more soluble in polar solvents like water. Nonpolar substances like oils are more soluble in nonpolar solvents.
4. Nature of the solvent - Polar solvents like water can dissolve ionic compounds and polar molecules through electrostatic interactions