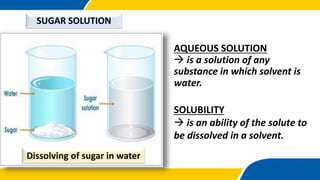
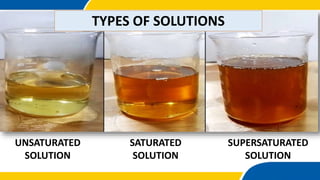
The document outlines a science lesson on types of solutions, focusing on definitions and properties of unsaturated, saturated, and supersaturated solutions. It includes objectives, examples, key terms, and questions to assess understanding, along with activities for students to demonstrate their knowledge. The lesson emphasizes the significance of solutions in daily life and includes tasks to perform experiments and engage students in further study.