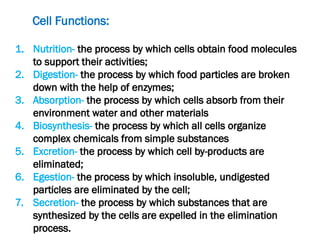
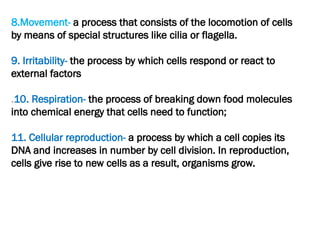
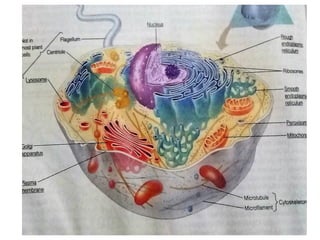
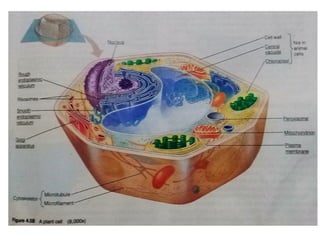
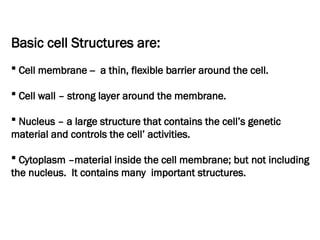
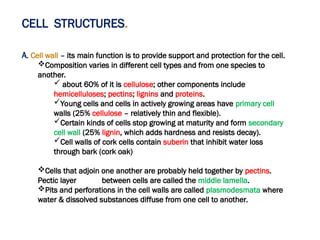
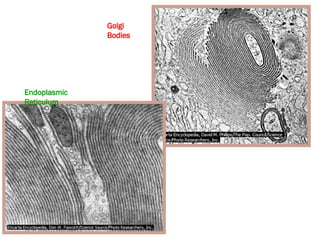
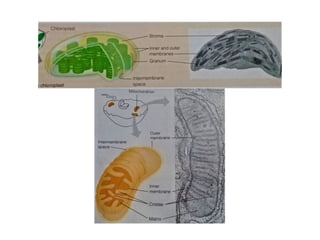
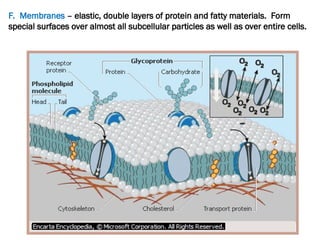
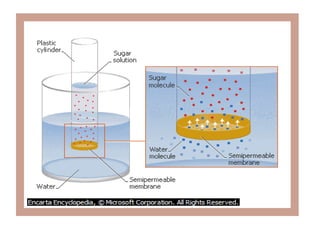
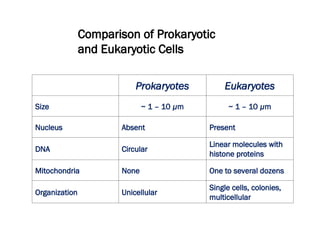
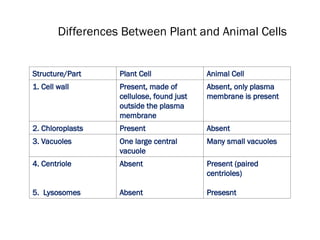
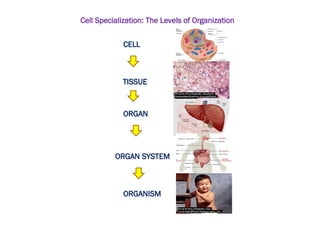
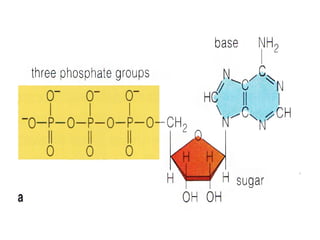
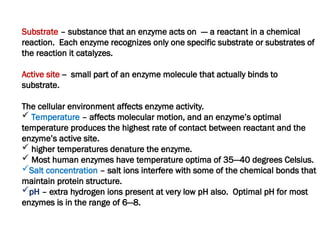
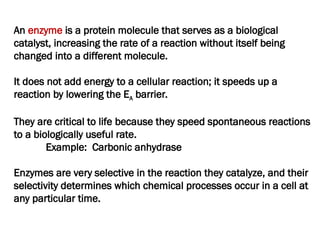
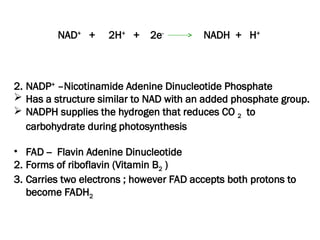
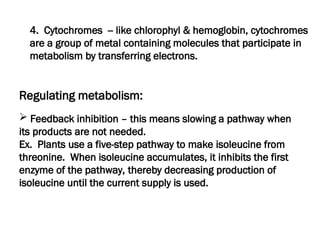
The document discusses cell structure and function, highlighting the importance of cells as the basic unit of life. It details the historical discoveries in cell biology, the components and functions of cells, including nutrition, respiration, and reproduction, as well as the various organelles within cells such as the nucleus, mitochondria, and ribosomes. The document also covers the principles of membrane transport, osmosis, and the differences between prokaryotic and eukaryotic cells.