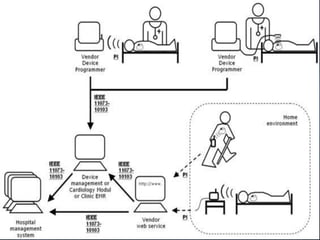
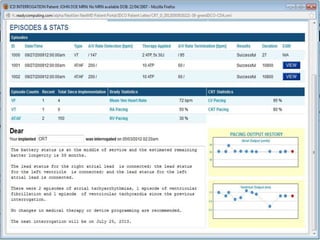
Patients increasingly seek access to data from their cardiac implantable electronic devices (CIEDs), necessitating standardization of terminology and processes for effective implementation. The document outlines a pilot project aimed at enhancing patient engagement through electronic messaging of remote device data, highlighting its potential to improve patient experiences and facilitate communication with healthcare providers. Currently, semi-automated notification systems are in beta testing, paving the way for greater transparency and understanding of device data among patients.