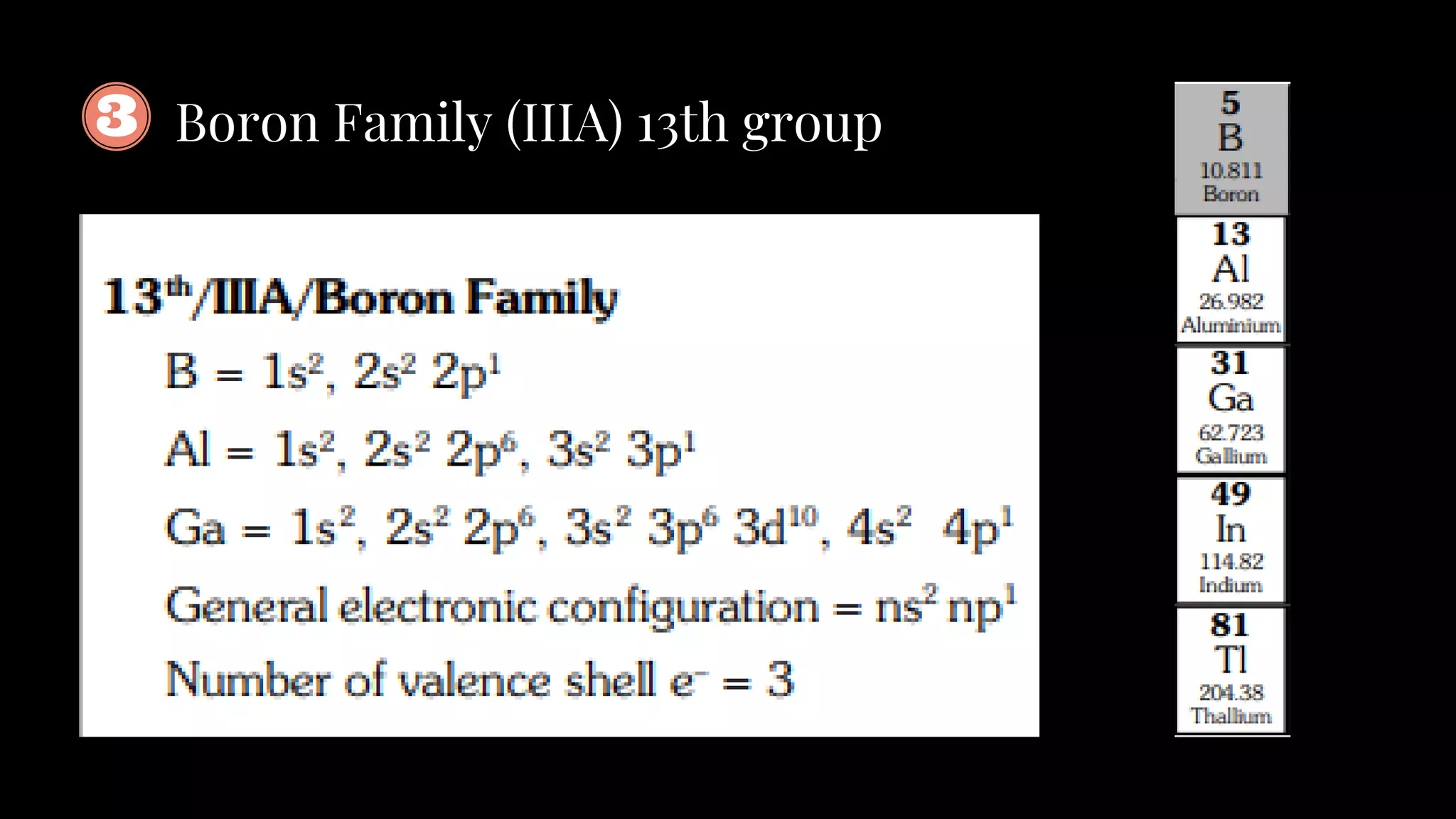
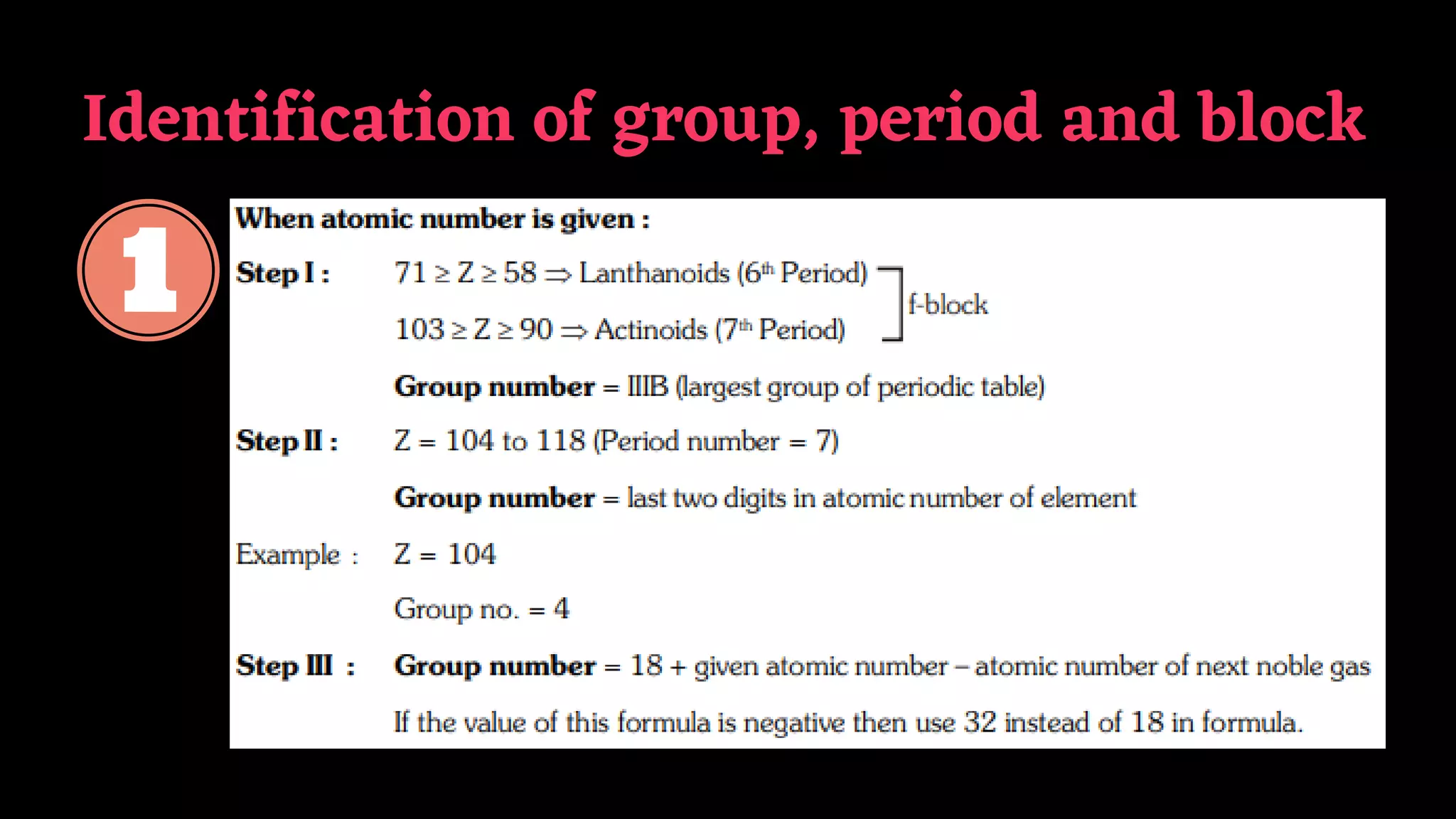
The document summarizes key aspects of the long form periodic table including its structure with 7 periods and 18 groups, the organization of elements into groups based on their outer electron configuration, and descriptions of the different groups including alkali metals, alkaline earth metals, and noble gases. It also provides some additional details on periodic trends, diagonal relationships between elements, and properties of typical elements.