Embed presentation
Download to read offline


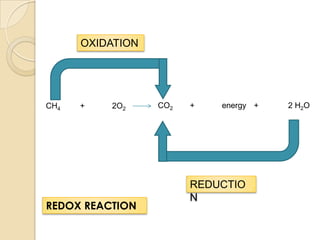

This document discusses chemical reactions and energy changes. It explains that chemical bonds contain potential energy and energy can be released or absorbed as bonds are broken or formed in reactions. It defines oxidation as the loss of electrons and reduction as the gain of electrons. As an example, it shows the redox reaction of methane and oxygen producing carbon dioxide, energy, and water, with methane undergoing oxidation and oxygen undergoing reduction.


