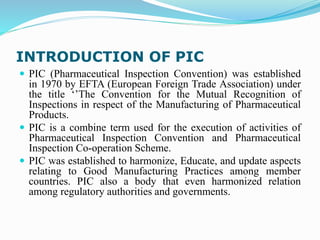
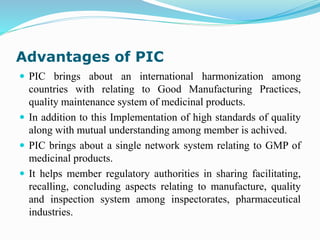
This document provides information about the US Food and Drug Administration (FDA). It describes the organization and responsibilities of the FDA, including protecting public health by regulating food, drugs, medical devices, and other products. It also summarizes key FDA centers like the Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research. The document introduces the Pharmaceutical Inspection Convention, its objectives to harmonize good manufacturing practices among countries.