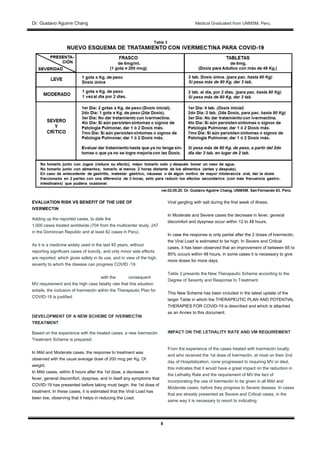
The document discusses evidence for the use of Ivermectin in treating COVID-19. It summarizes a study of 704 COVID-19 patients treated with Ivermectin that found their case fatality rate was 6.1 times lower than those not treated with Ivermectin. It also describes reports from the Dominican Republic of 247 COVID-19 patients treated with Ivermectin who all showed favorable responses with no reported deaths. The document concludes that given Ivermectin's safety profile, it should be formally included in the first line of treatment for COVID-19.