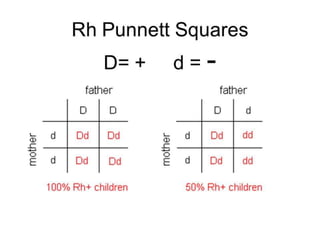
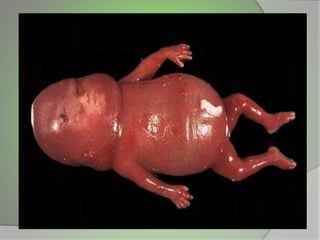
The document discusses the Rhesus factor and its implications for pregnancy, particularly focusing on Rh incompatibility between an Rh-negative mother and an Rh-positive fetus. It details the mechanisms of sensitization, the resulting hemolytic disease of the fetus and newborn (HDFN), and the various clinical manifestations and preventative measures. The document also outlines diagnosis, delivery planning, and the methods of delivery in cases of Rh incompatibility.