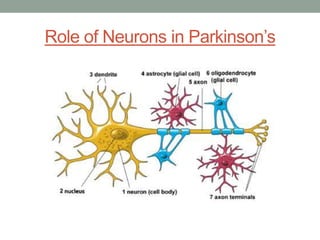
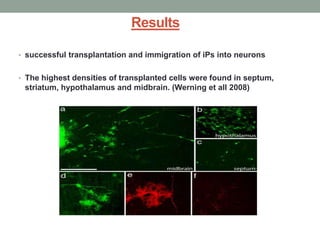
This document discusses the potential use of induced pluripotent stem cells (iPSCs) derived from fibroblasts for treating Parkinson's disease. It summarizes that iPSCs can be generated from adult fibroblasts through retroviral transduction of specific transcription factors. One study discussed found that when neurons derived from reprogrammed mouse fibroblasts were transplanted into a rat model of Parkinson's, they functionally integrated into the brain and improved motor symptoms. The document concludes that while iPSC-derived cells show promise for treating Parkinson's, more research is still needed to address safety issues before applying this technique to humans.