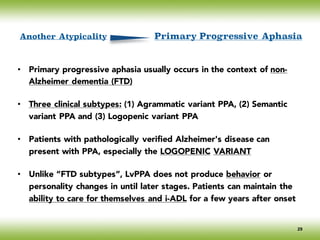
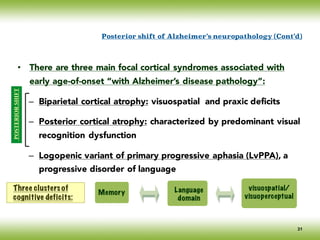
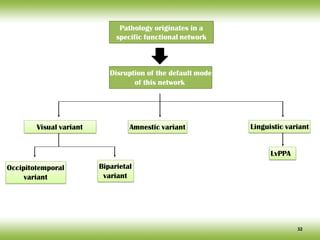
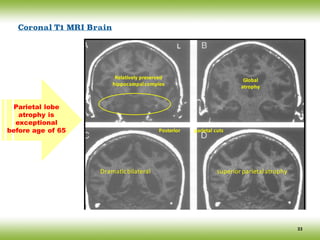
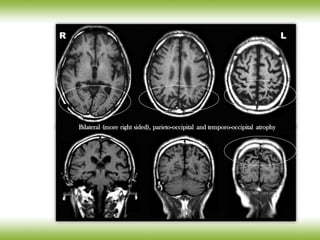
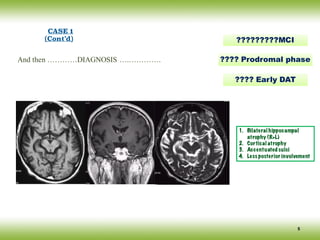
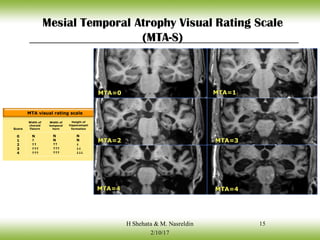
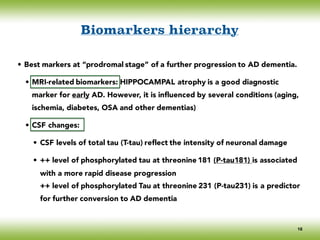
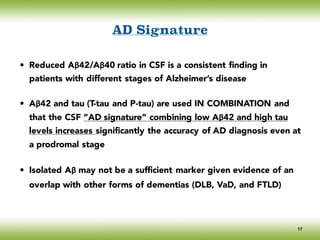
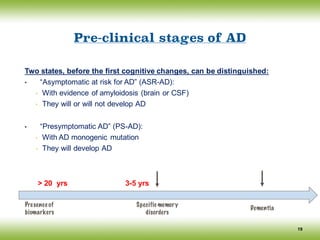
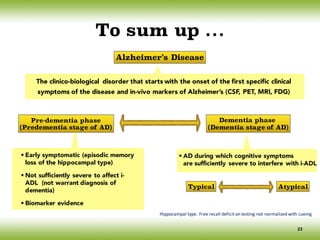
The document discusses neurocognitive disorders, focusing on new diagnostic criteria for Alzheimer's disease, including early and prodromal phases, as well as validated biomarkers. It highlights the need for updated guidelines that align with current clinical practices, detailing different types of neurocognitive disorders and the significance of biomarker evidence in diagnosing Alzheimer’s. Additionally, it describes atypical forms of Alzheimer's pathology and their unique clinical presentations, emphasizing the evolution and understanding of the disease's characteristics.






![Diagnostic and statistical manual (DSM-5)
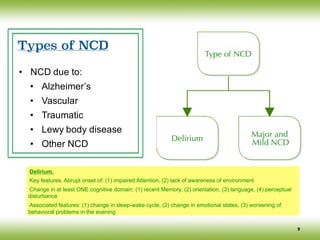
• Major NCD
• Significant cognitive decline [2 or more cognitive domains impaired]
• Interfere with independence [impaired IADLs]
• Not due to delirium
• Not due to other mental disorder
• Mild NCD
• Moderate Cognitive Decline [1 or more cognitive domains impaired]
• NOT Interfere with independence [IADLs intact]
• Not due to delirium
• Not due to other mental disorder
10
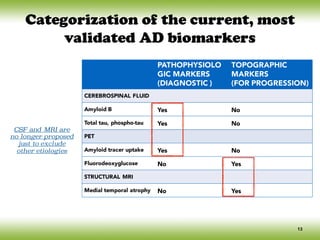
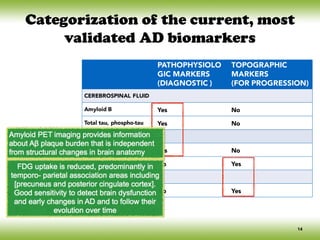
Insidious onset & gradual progression](https://image.slidesharecdn.com/cnc-18alzheimer-170210201201/85/Alzheimer-Disease-10-320.jpg)
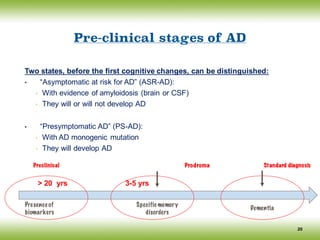
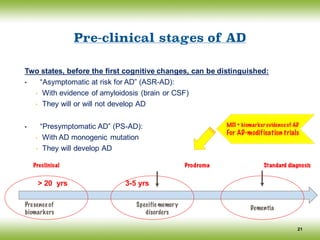
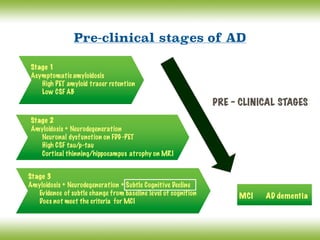
![Conceptual framework changes of AD
The discovery of biomarkers as surrogate markers of the underlying pathological changes,
led an international work group (IWG) to propose a new conceptual framework for AD in
2007 and [Lexicon revision in 2010] (Dubois, Feldman et al).
The main advances of the IWG/DUBOIS Criteria:
12
Consider AD as encompassing predementia and dementia stages
Which includes atypical subtypes of AD
AD is defined as a clinico- biological (rather than a clinico-pathological) entity that
can be recognized before the onset of the dementia syndrome, on the basis of:
(i) A specific clinical phenotype (amnestic syndrome of the hippocampal type), and
(ii) Supportive biomarkers evidence
Differentiate pathophysiological and topographical biomarkers
Isolate and define a prodromal stage of the disease
Define biomarker positive cognitively normal subjects as ‘asymptomatic at risk for AD’
(their risk to clinical conversion is not known)
Define monogenic mutation careers as ‘presymptomatic’
(the risk to clinical conversion is certain)](https://image.slidesharecdn.com/cnc-18alzheimer-170210201201/85/Alzheimer-Disease-12-320.jpg)
![• 70 % of subjects with MCI ------ AD within 5 years [10% - 15% per
year]
• Normal subjects develop AD at a rate of [1% - 2% per year]
• Types: Amnestic, Multiple domains, Single non-memory domain
(executive functions)
Normal
MCI
DAT
Cognitive Continuum
Mild Cognitive Impairment
18](https://image.slidesharecdn.com/cnc-18alzheimer-170210201201/85/Alzheimer-Disease-18-320.jpg)
![Typical Alzheimer’s disease
24
* Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–59
Initial involvement of the
entorhinal cortex, and
medial temporal structures
De-afferentiation
of hippocampus
Spreads to iso &
neocortical association areas
Amnestic syndrome of the
hippocampal type
Episodic antero-grade
memory impairment
Typical initial core pattern of
cognitive changes of AD
Executive dysfunction,
Language, Praxis,
& complex visual processing
impairment
Cueing is a measure of the associative
function of the hippocampus
In-vivopositive biomarkers of
Alzheimer’s pathology
Typical regional brain pathology of AD [Braak and Braak*] revealed:
D-RELATED NEUROFIBRILLARY CHANGES 275
transentorhinal stages
I
/STAGE I // ~
region
.o,o:.o....,;ZU ,
t
X
(STAGE II II I - ..
~/~--~ •
I
limbic stages
l
STAGEIII// "~ /" ~
~ ?..°o - i ,
~..:.,, ...........,. . , .
. ....
isocortical stages
t t
FIG. 4. Summary diagram of neurofibrillarychanges seen in the hippocampal formation, entorhinal
and transentorhinal regions, and in the adjoiningtemporal isocortex. Note the development of changes
I
/STAGE I // ~
region
.o,o:.o....,;ZU ,
t
X
(STAGE II II I - ..
~/~--~ •
I
limbic stages
l
STAGEIII// "~ /" ~
~ ?..°o - i ,
~..:.,, ...........,. . , .
. ....
isocortical stages
t t
FIG. 4. Summary diagram of neurofibrillarychanges seen in the hippocampal formation, entorhinal
and transentorhinal regions, and in the adjoining temporal isocortex. Note the development of changes
from stage I to stage VI. The arrows point to leading characteristics. CAI: first sector of the Ammon's
horn, parasub: parasubiculum, presubic: presubiculum; transentorhin, region.: transentorhinal region;
entorhin.region: entorhinal region; temp. isocortex: temporal isocortex; reproduced with permission
from (12).
characteristic brain lesions, stage III or IV cases are considered
to represent incipient AD (12,14,15).
TRANSENTORHINAL STAGES 1 AND II
In the most mildly affected brains involvement only of layer
Preu is consistently displayed and this change is initially con-
fined to the transentorhinal region (Fig. le and Fig. 4). Clini-
cally, these stages do not present with any cognitive impairments
(6,18,31). As in many other neurodegenerative diseases, there
is a period of time in which NFTs and NTs slowly and gradu-
ally develop at their predilection sites. However, the brain
change remains below the ~.hreshold which produces clinical
symptoms (12,14,15).
SITUATION BEFORE THE FORMAT
OF NEUROFIBRILLARY CHANGE
Application of specific immunocytochem
offers the possibilityof going back before stag
examination of the neuronal cytoskeletal chang
actual formation of NFTs and NTs, This kin
carried out in transentorhinal region and in b
atively young individuals. Before or during s
is generally devoid of A4-amyloid deposits, neu
cular changes, or other pathologic lesions. This
tunity to study these early events in the absenc
Sensitive and specific antibodies (AT8) reactin
mally phosphorylated tau protein (36) display
appear rather abruptly in the transentorhinal](https://image.slidesharecdn.com/cnc-18alzheimer-170210201201/85/Alzheimer-Disease-24-320.jpg)

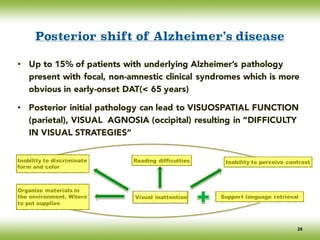
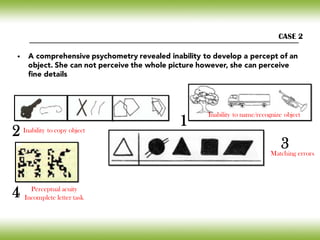
![• The tasks of identifying and locating objects are located in different
cortical areas
• Neuronal circuits that project from [striate cortex]
Posterior shift of Alzheimer’s neuropathology
The ventral pathway acts in the
visual recognition of objects
“STIMULUS RECOGNITION”
The dorsal pathway acts in the
recognition of space (visual
guidance of movement)
“STIMULUS LOCALIZATION “
STS
Striate cortex
ITG
27](https://image.slidesharecdn.com/cnc-18alzheimer-170210201201/85/Alzheimer-Disease-27-320.jpg)