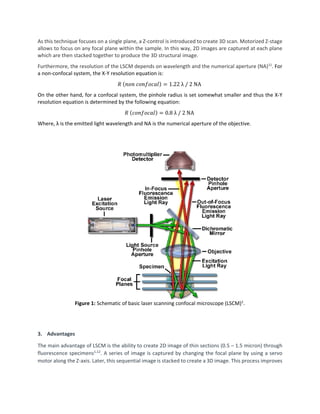
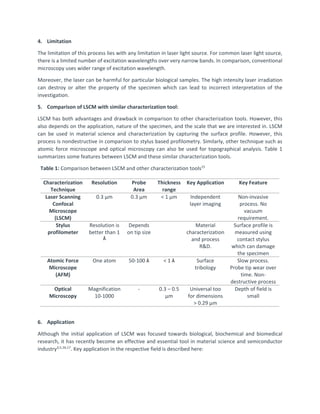
This document provides an overview of laser scanning confocal microscopy (LSCM). It discusses the working principle of LSCM, highlighting how it uses pinholes and laser excitation to optically section samples. Advantages include high resolution 3D imaging without physical sectioning. Applications described include biology, materials science, and semiconductor fabrication. LSCM is presented as a valuable non-destructive characterization tool across many fields due to its high resolution and ability to image living samples.