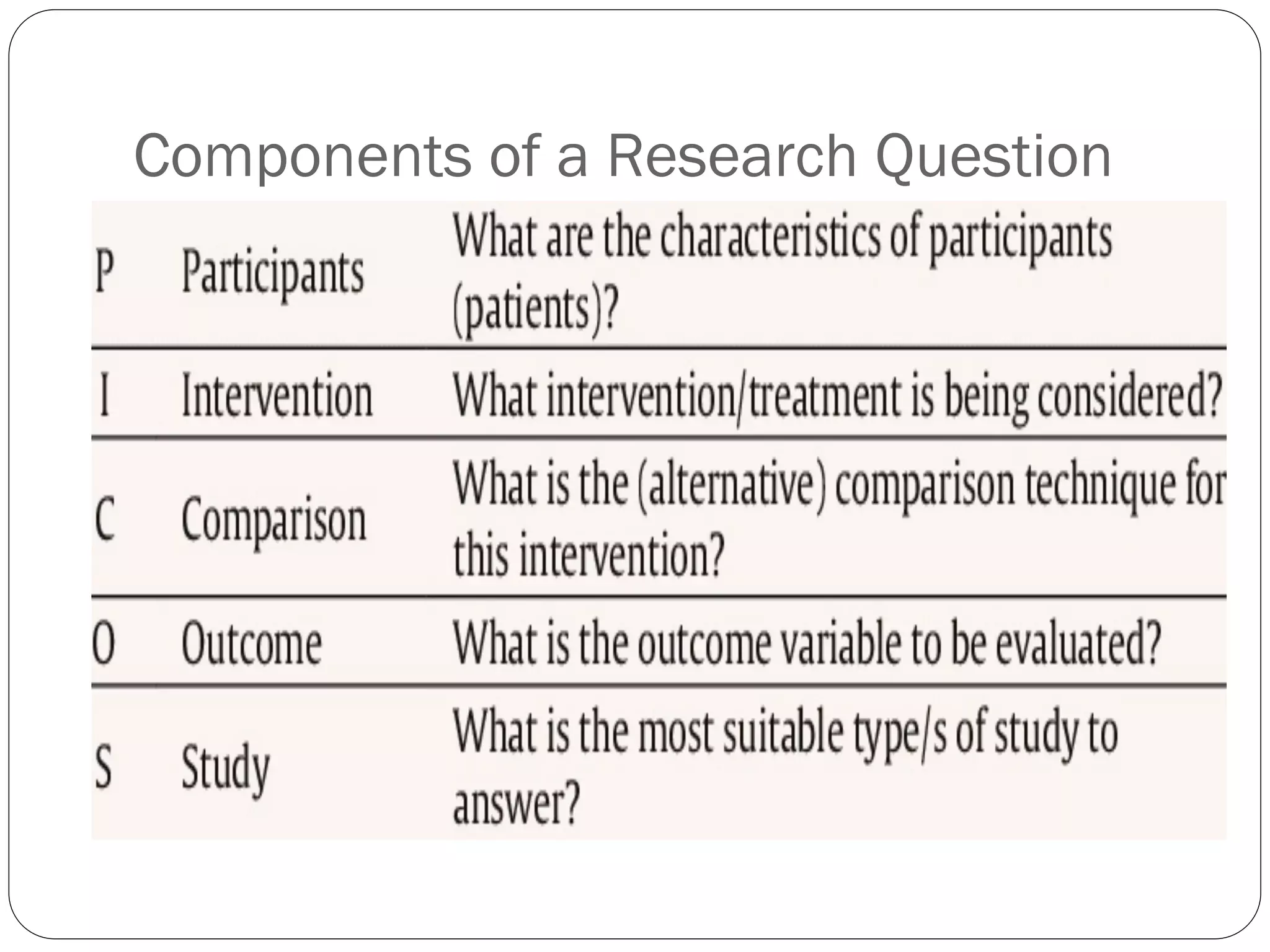
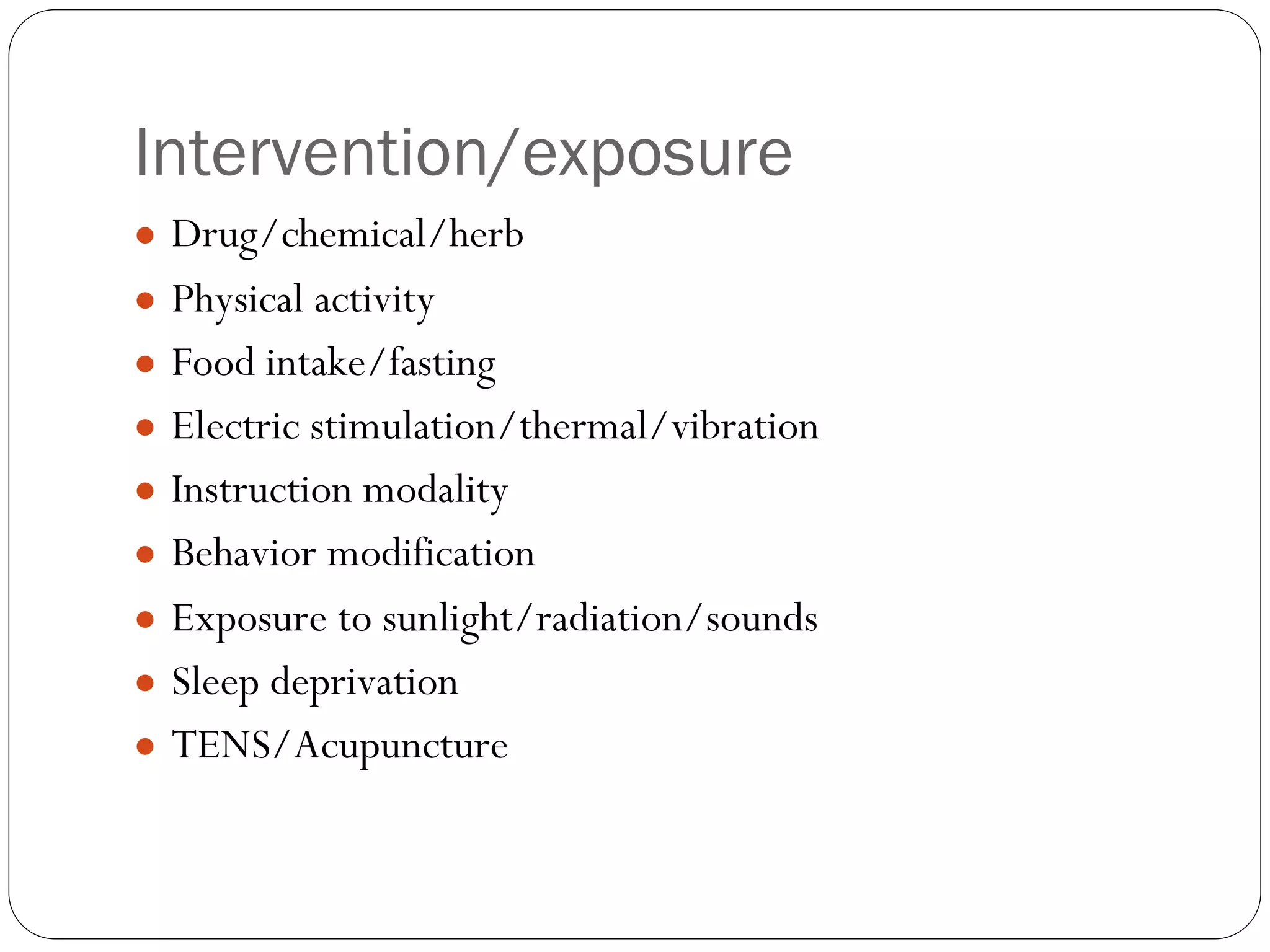
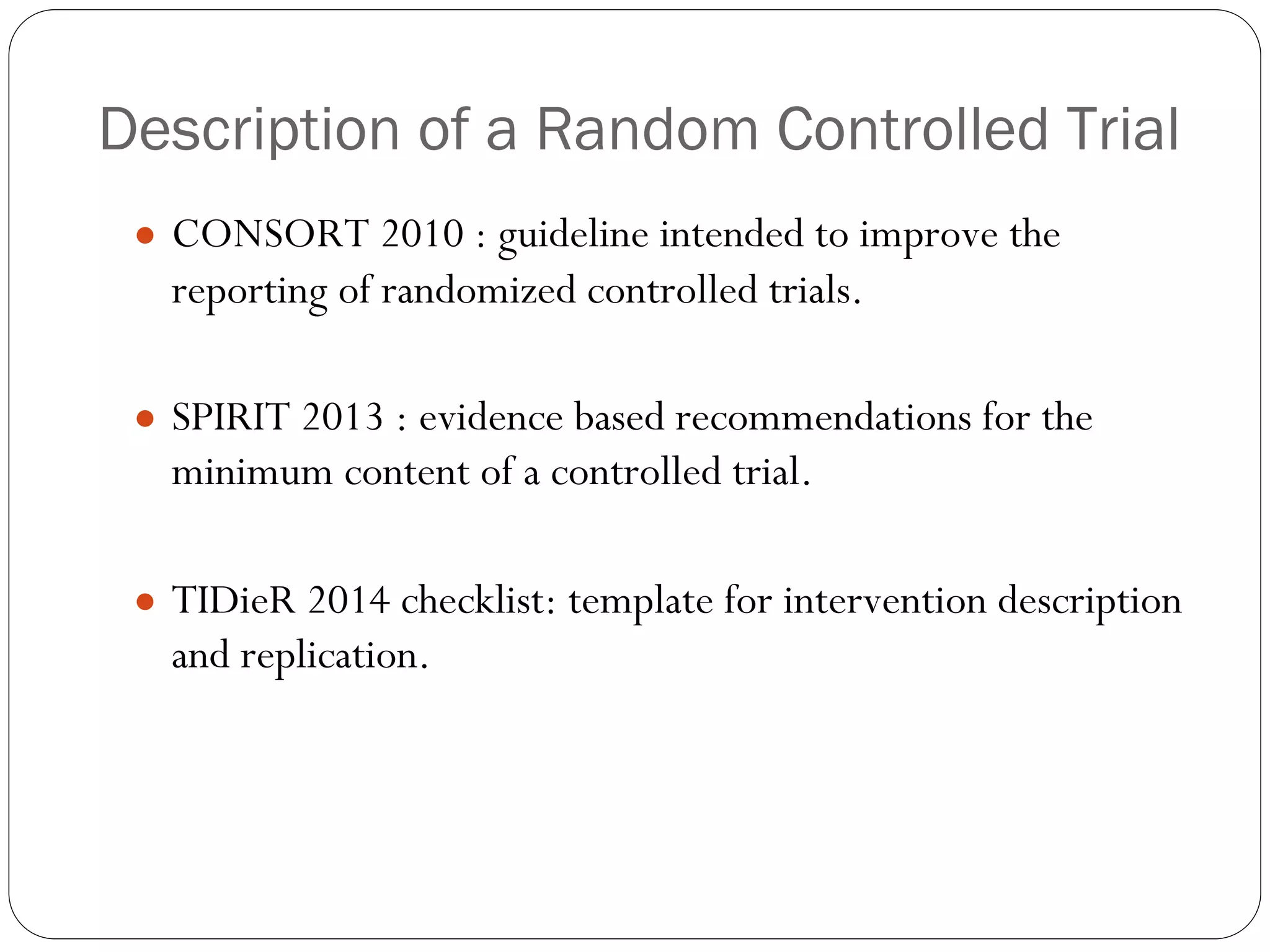
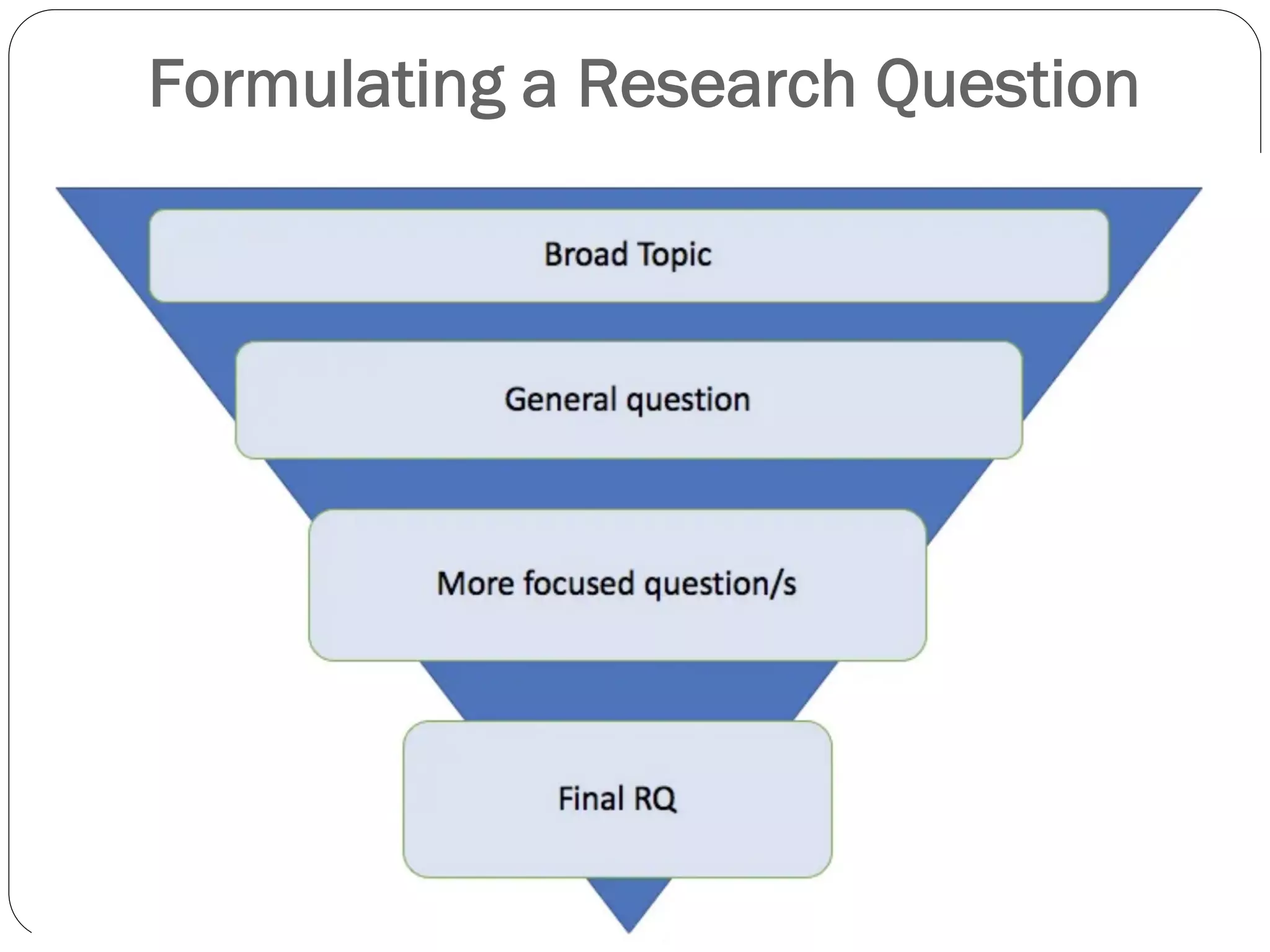
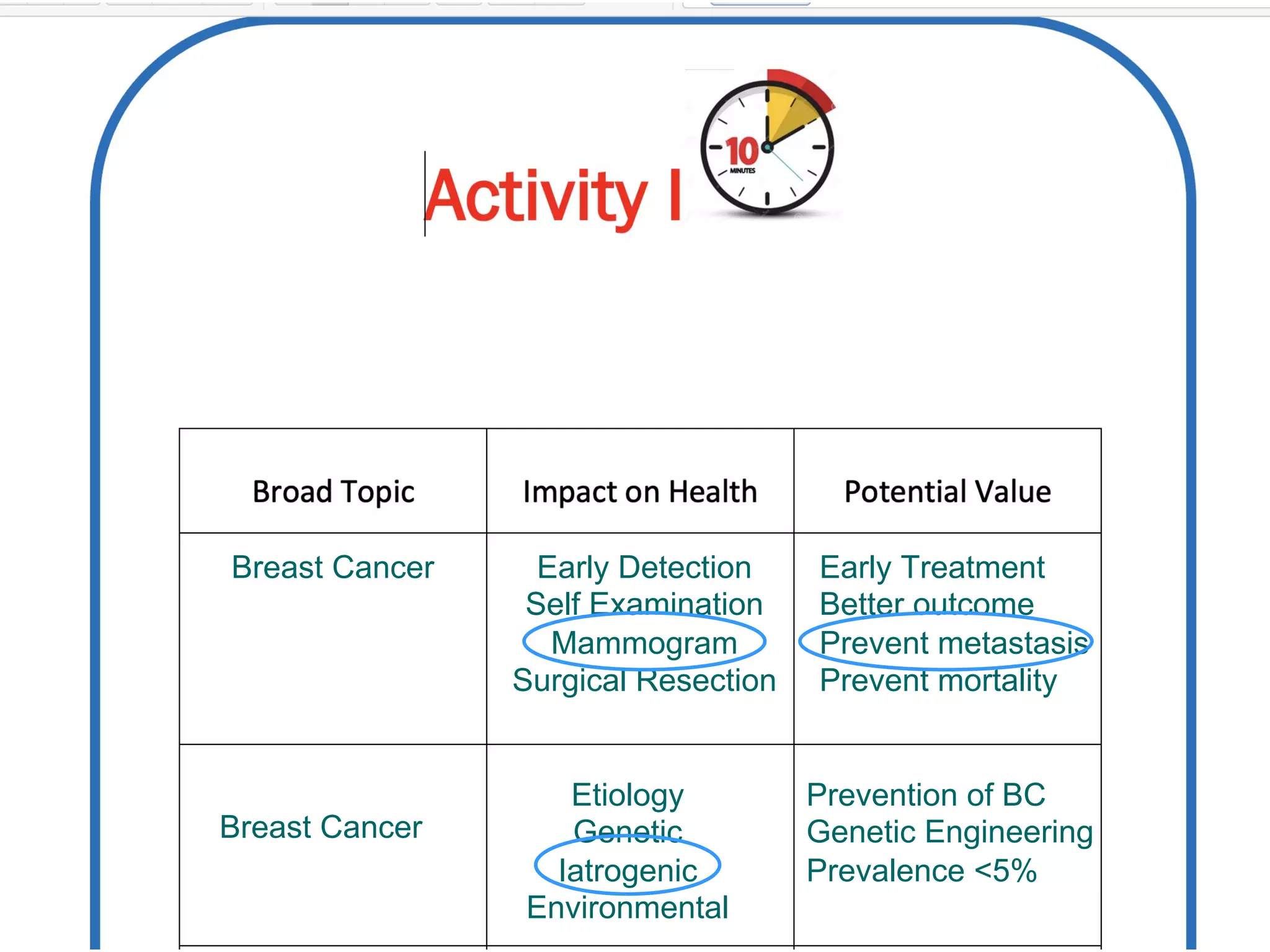
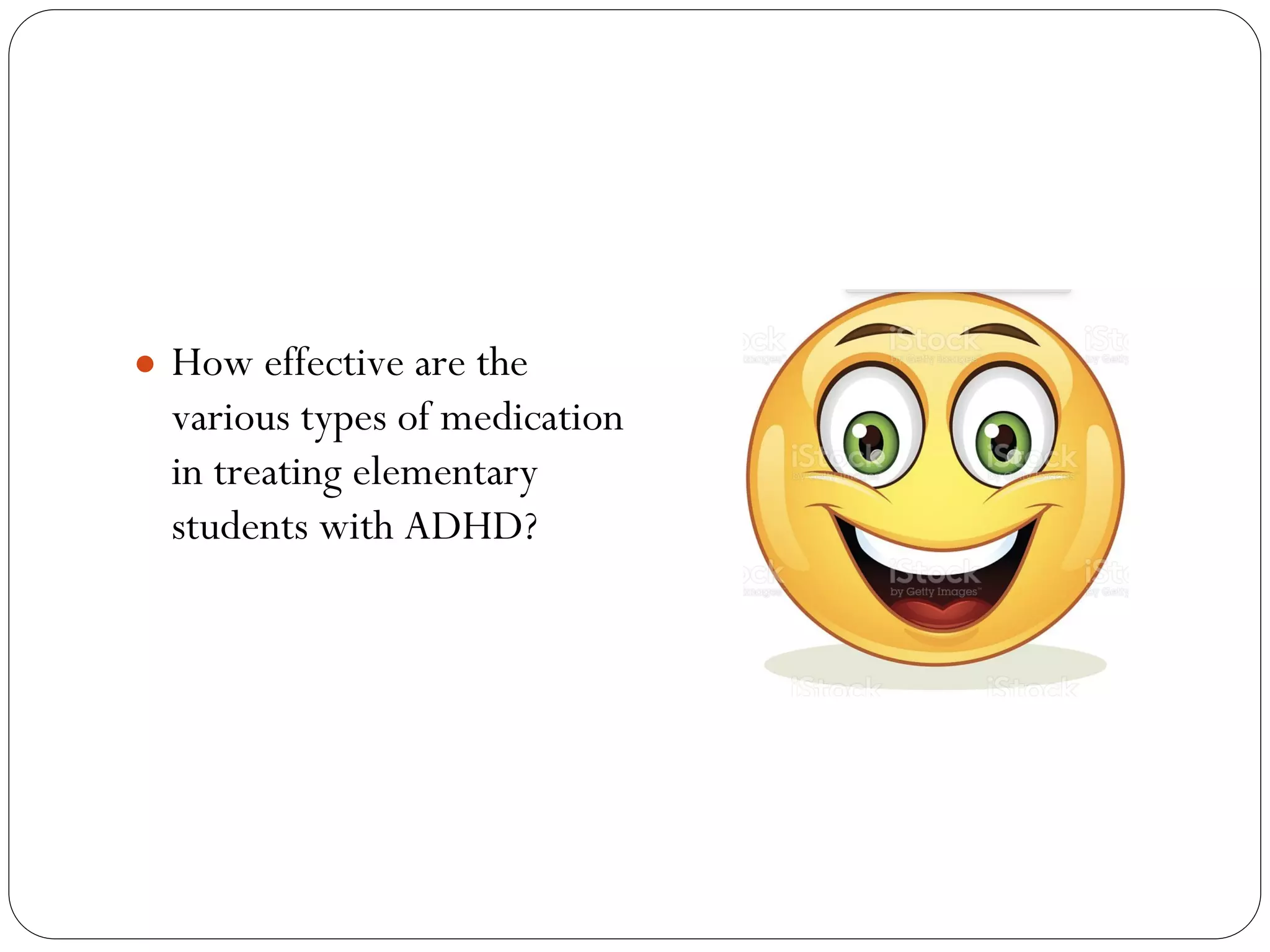
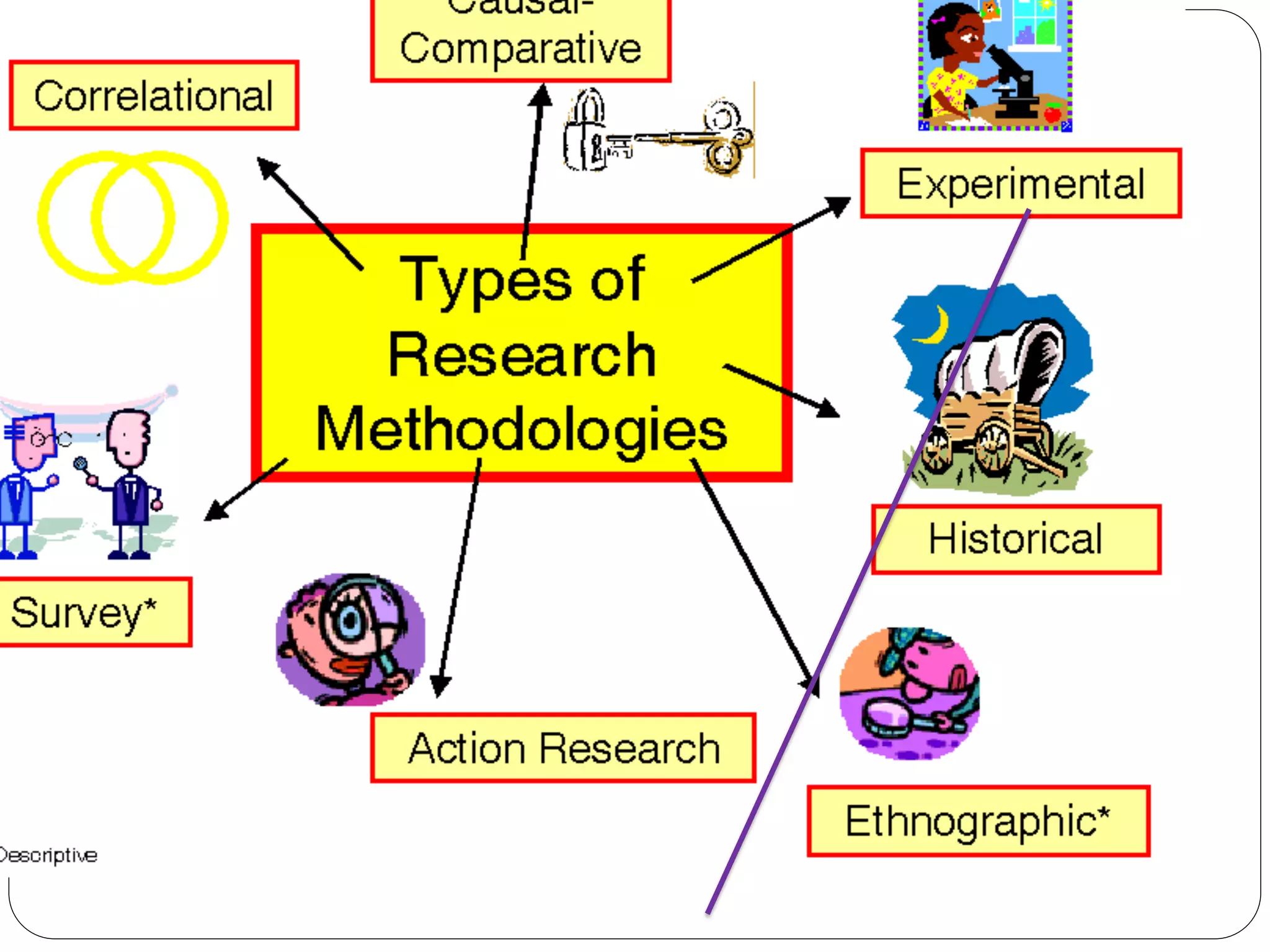
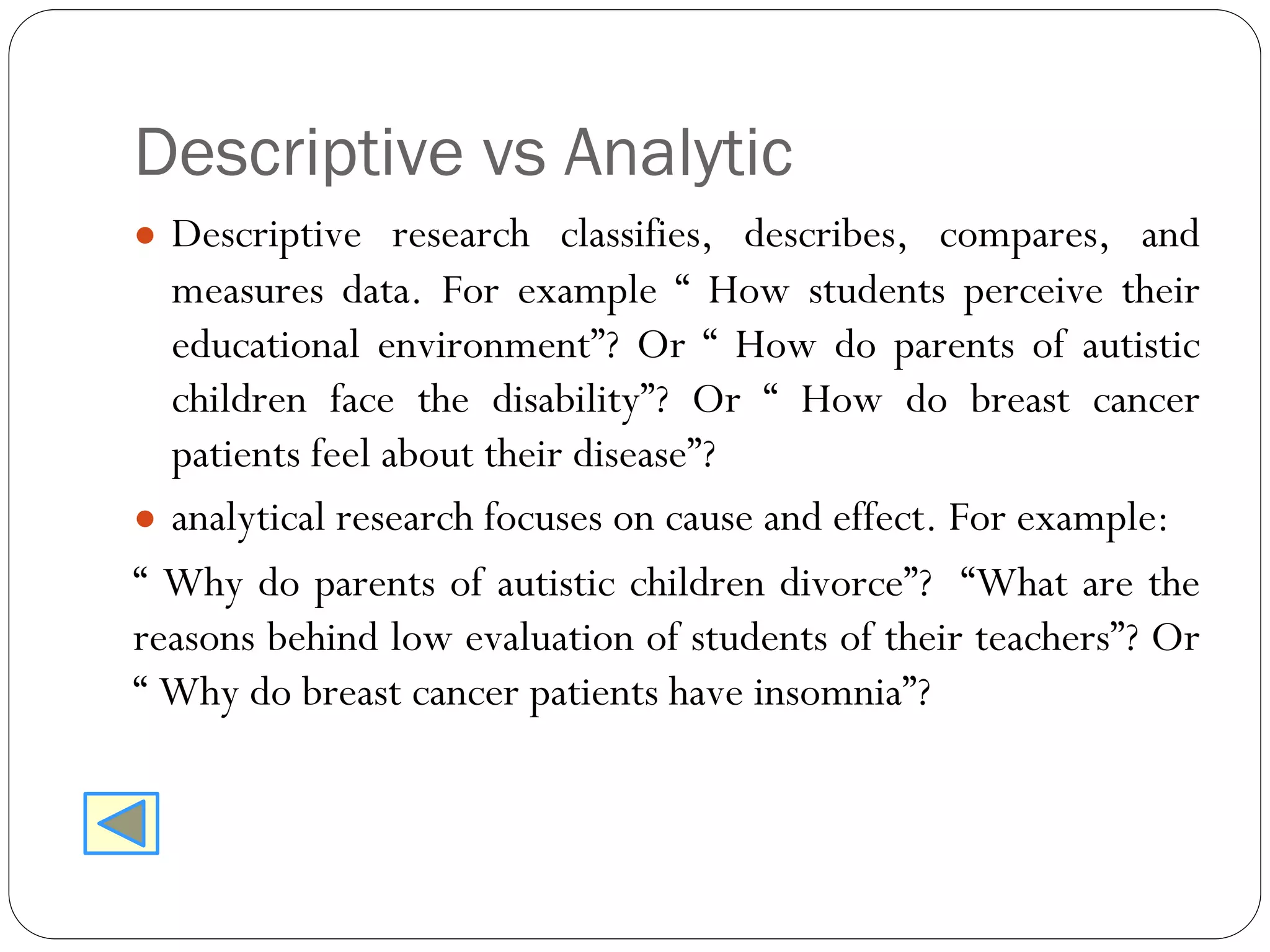
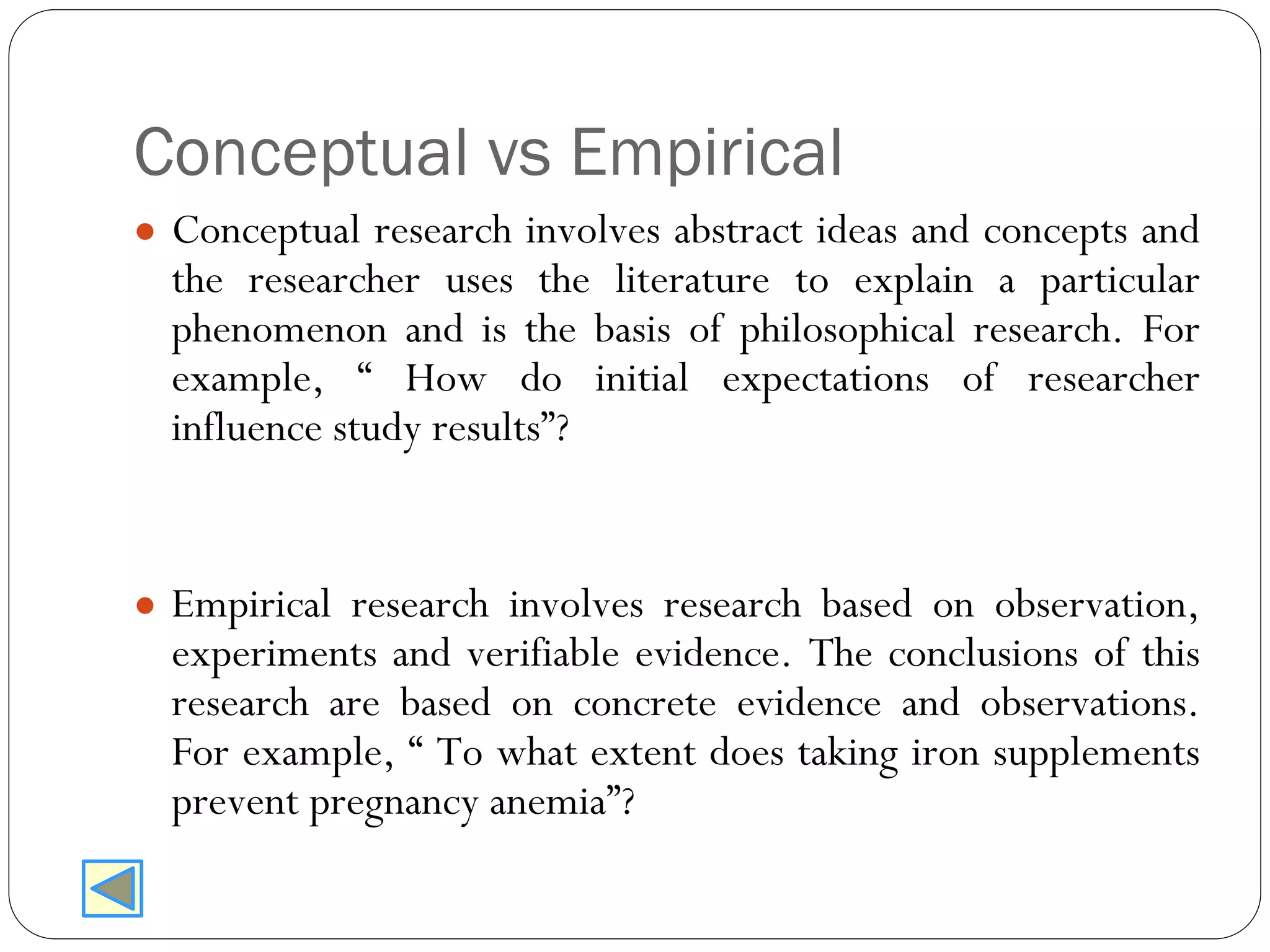
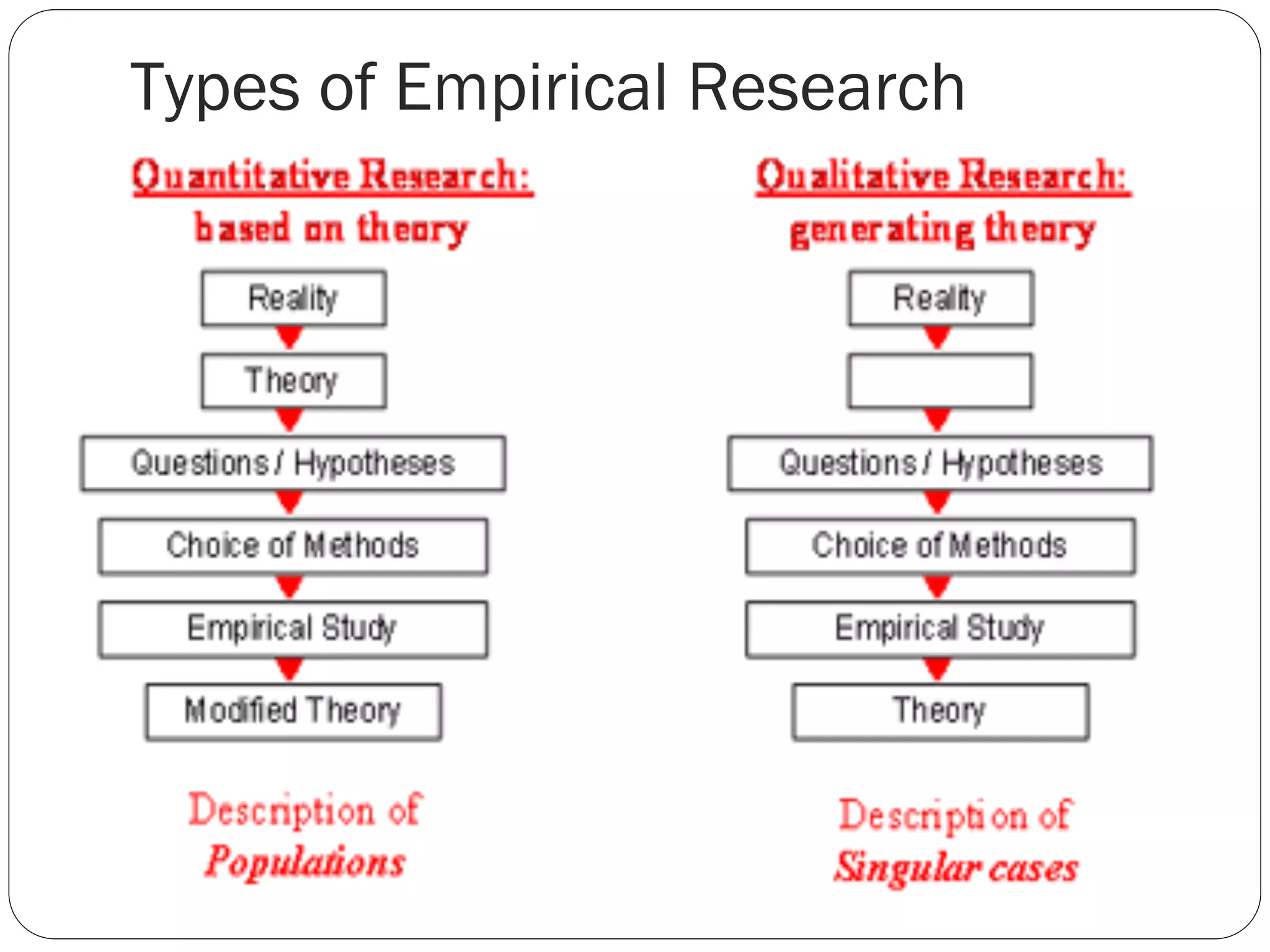
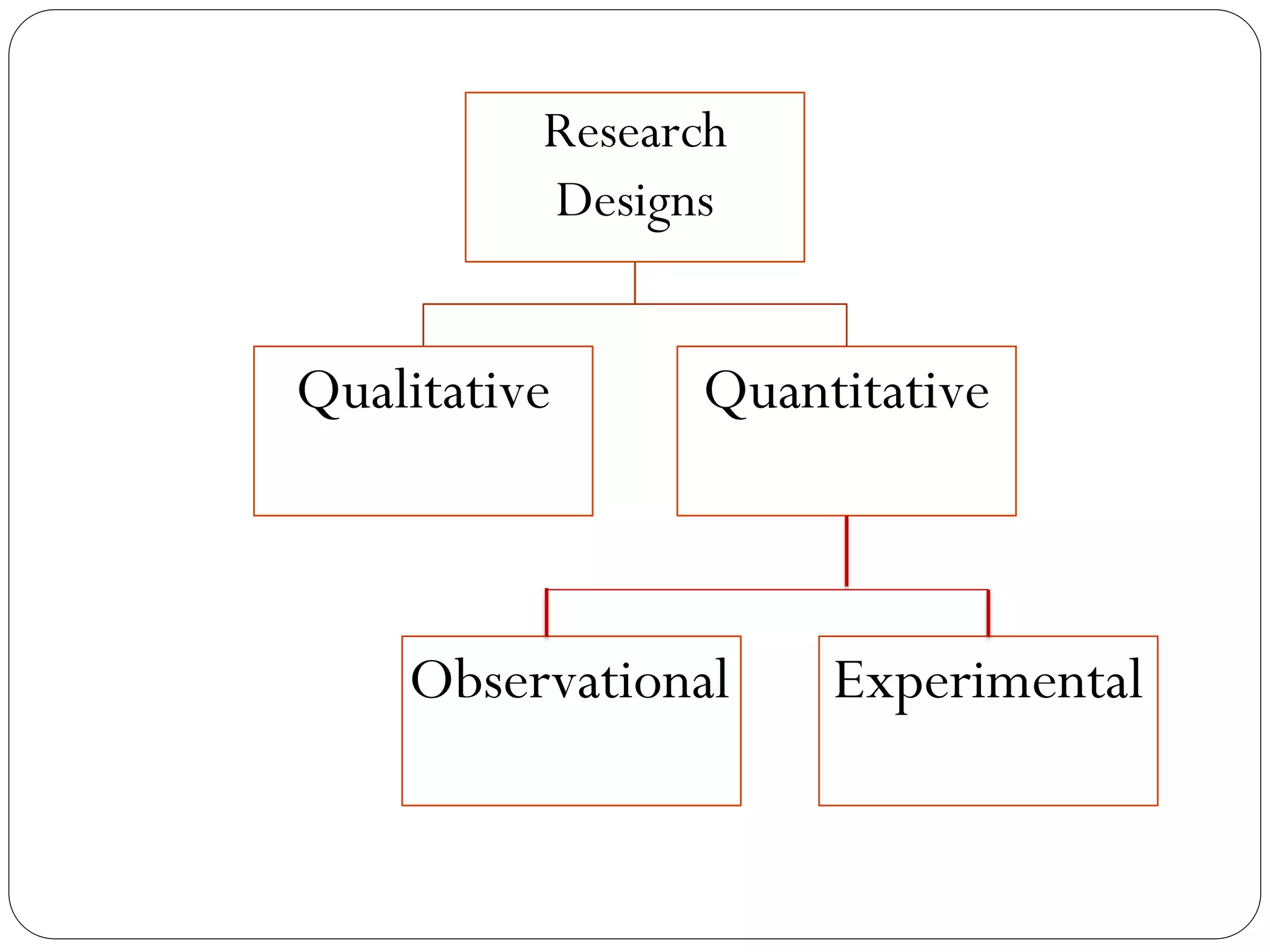
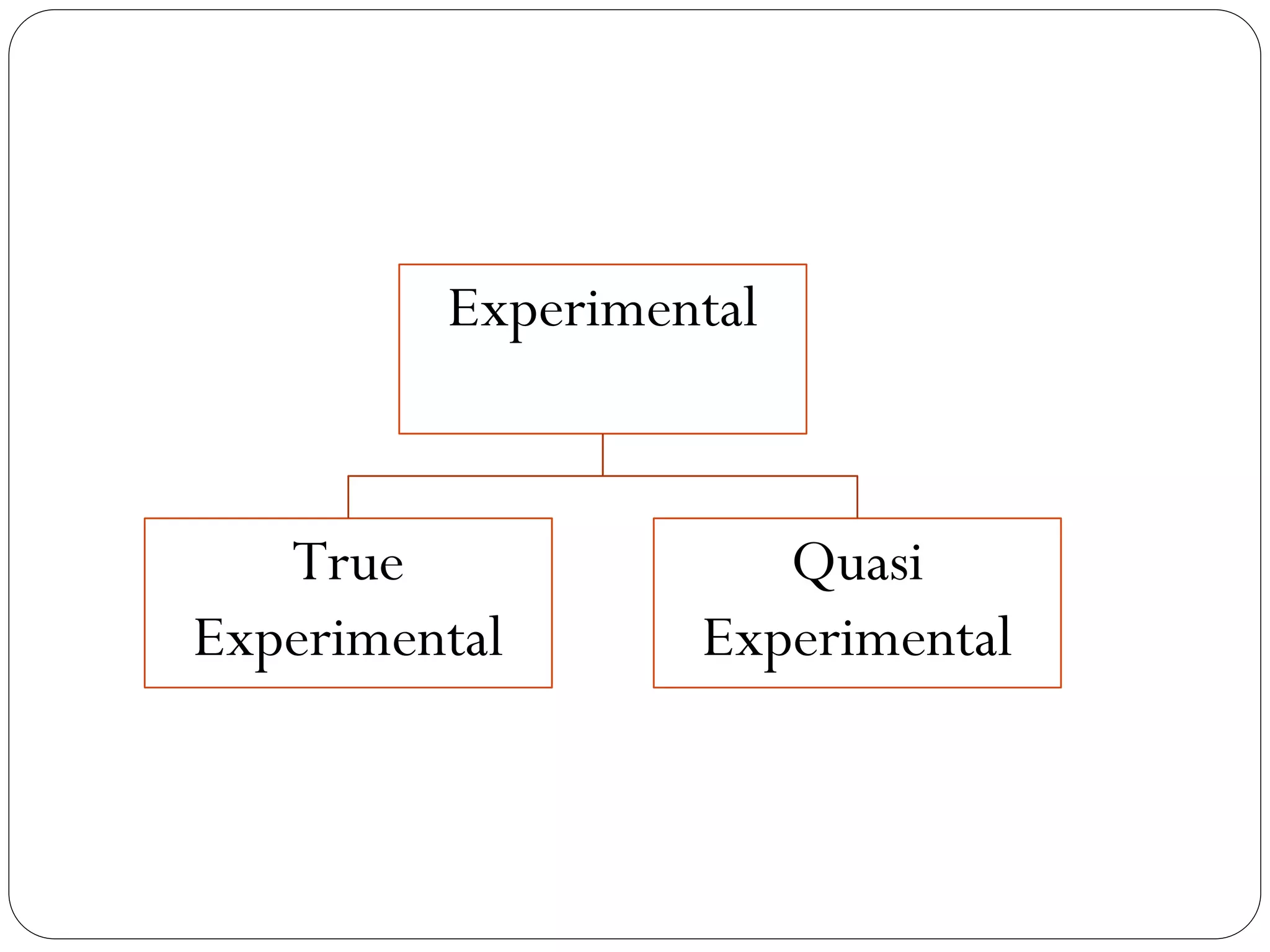
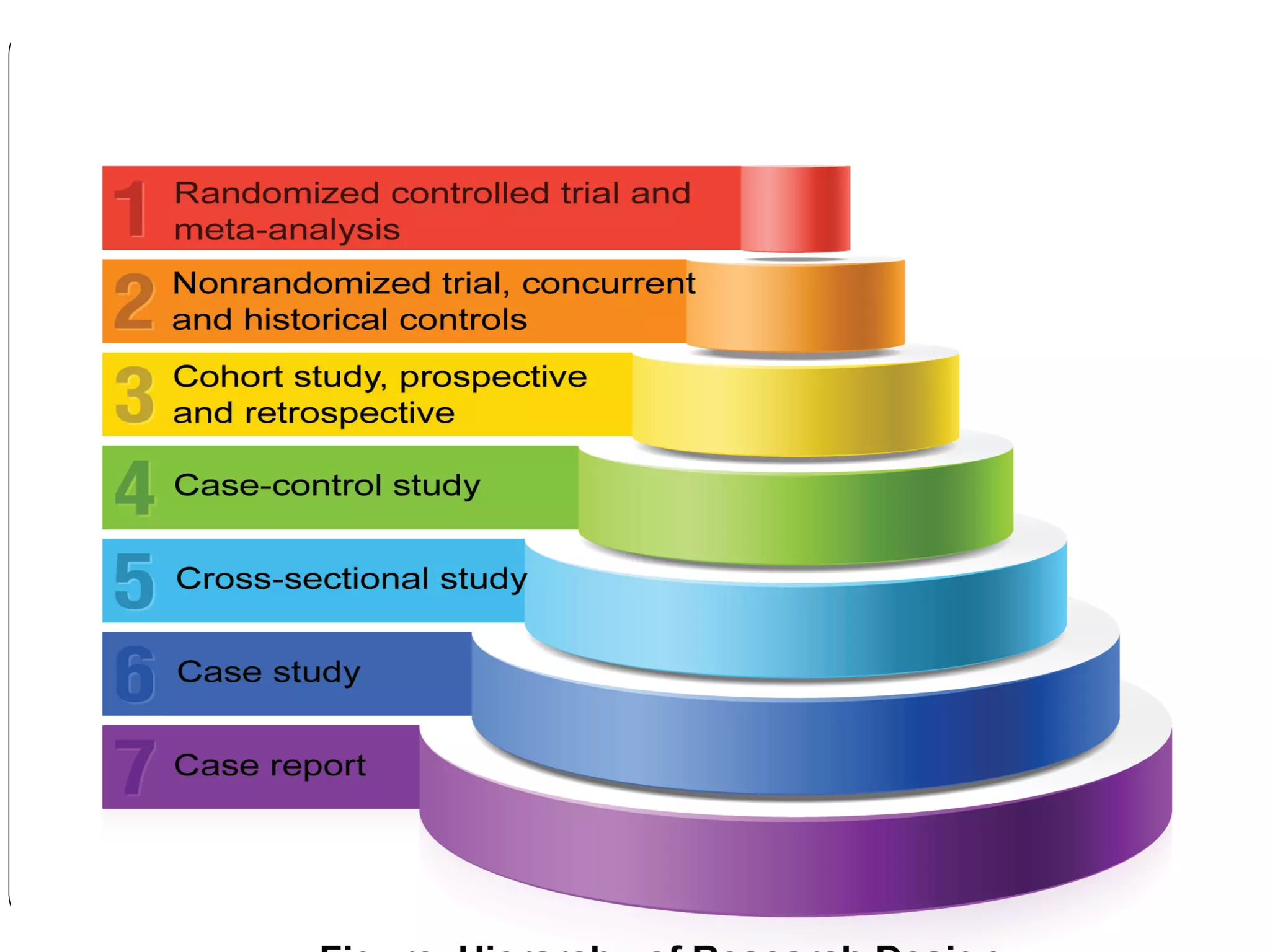
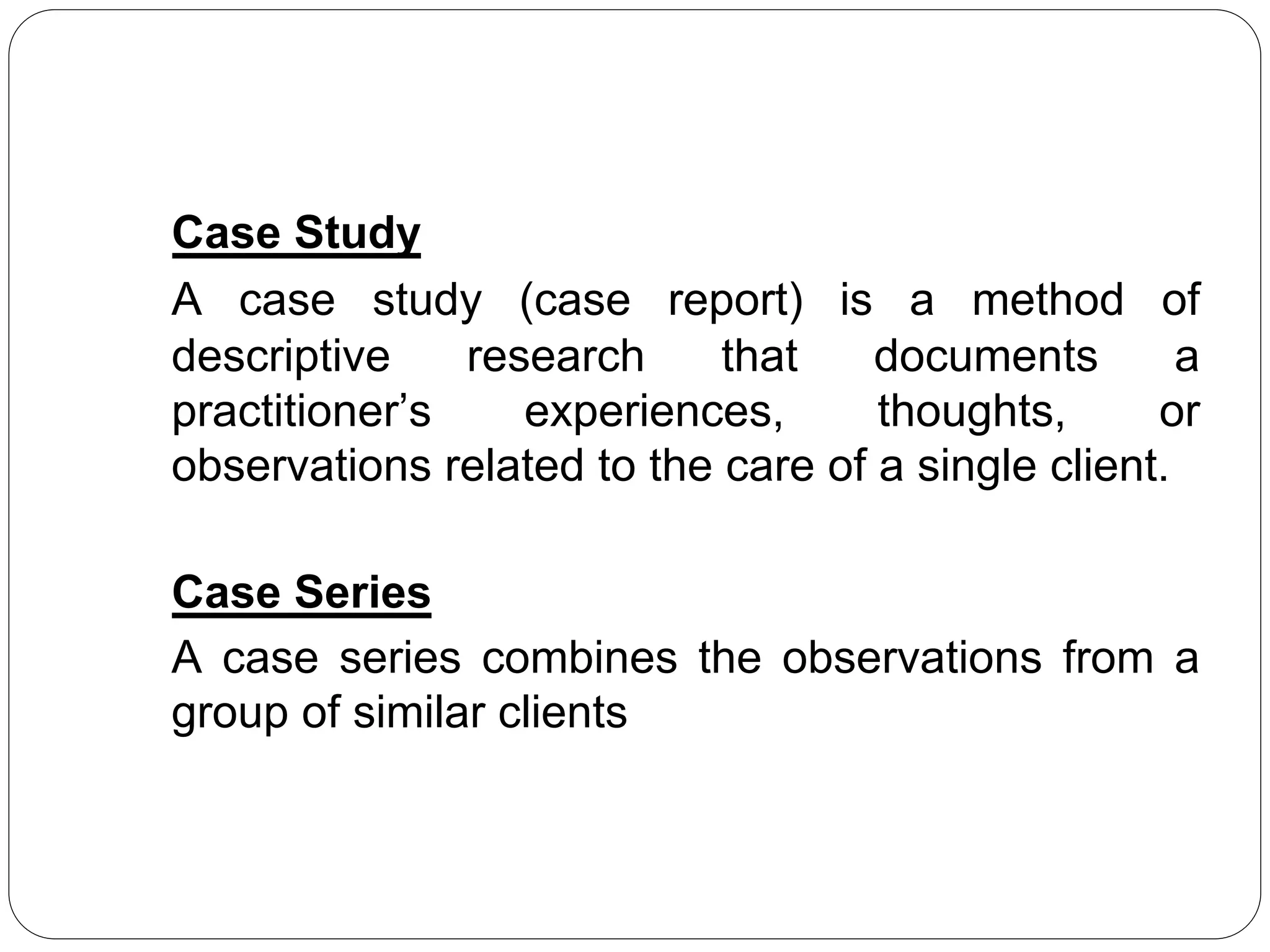
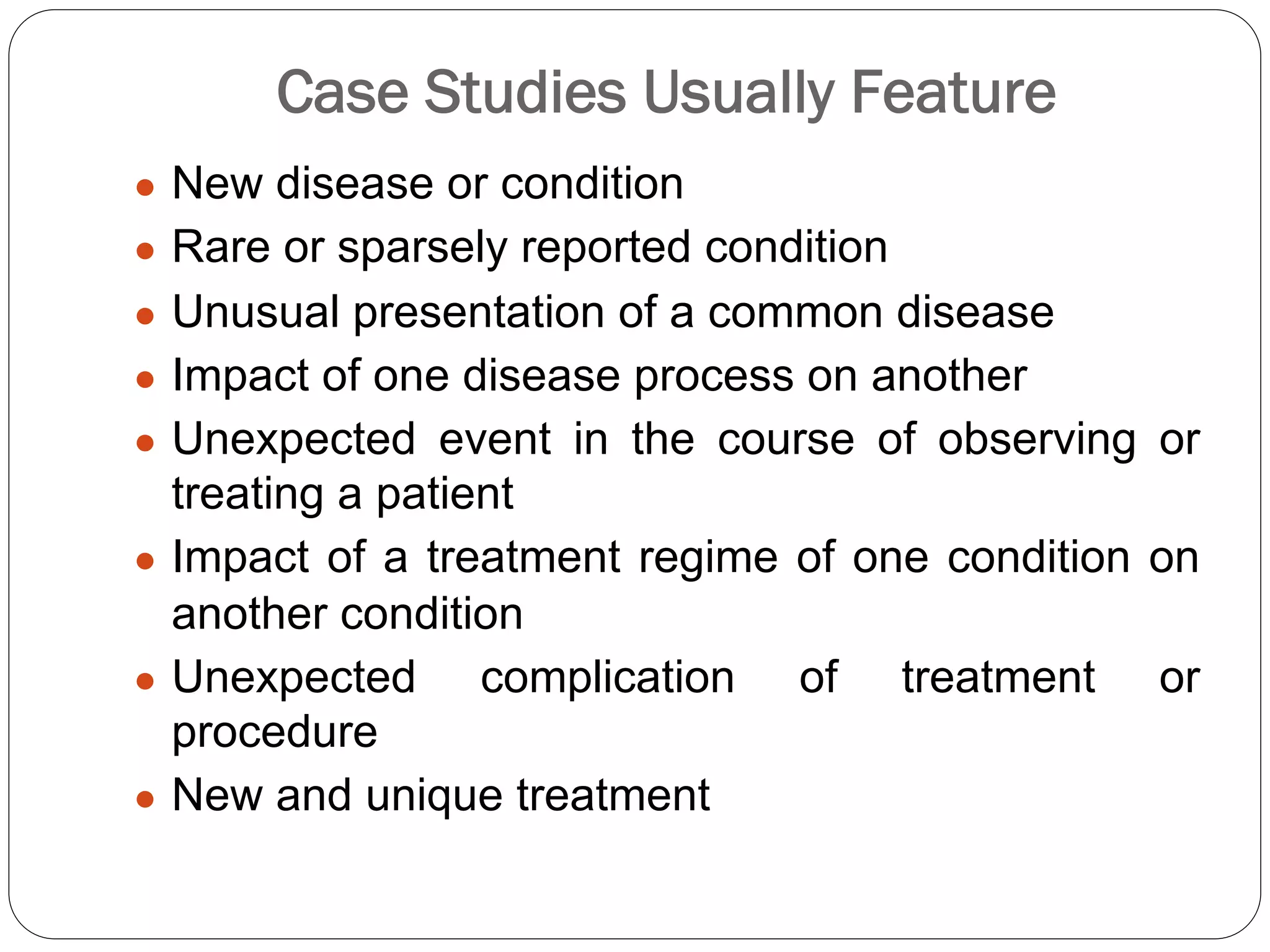
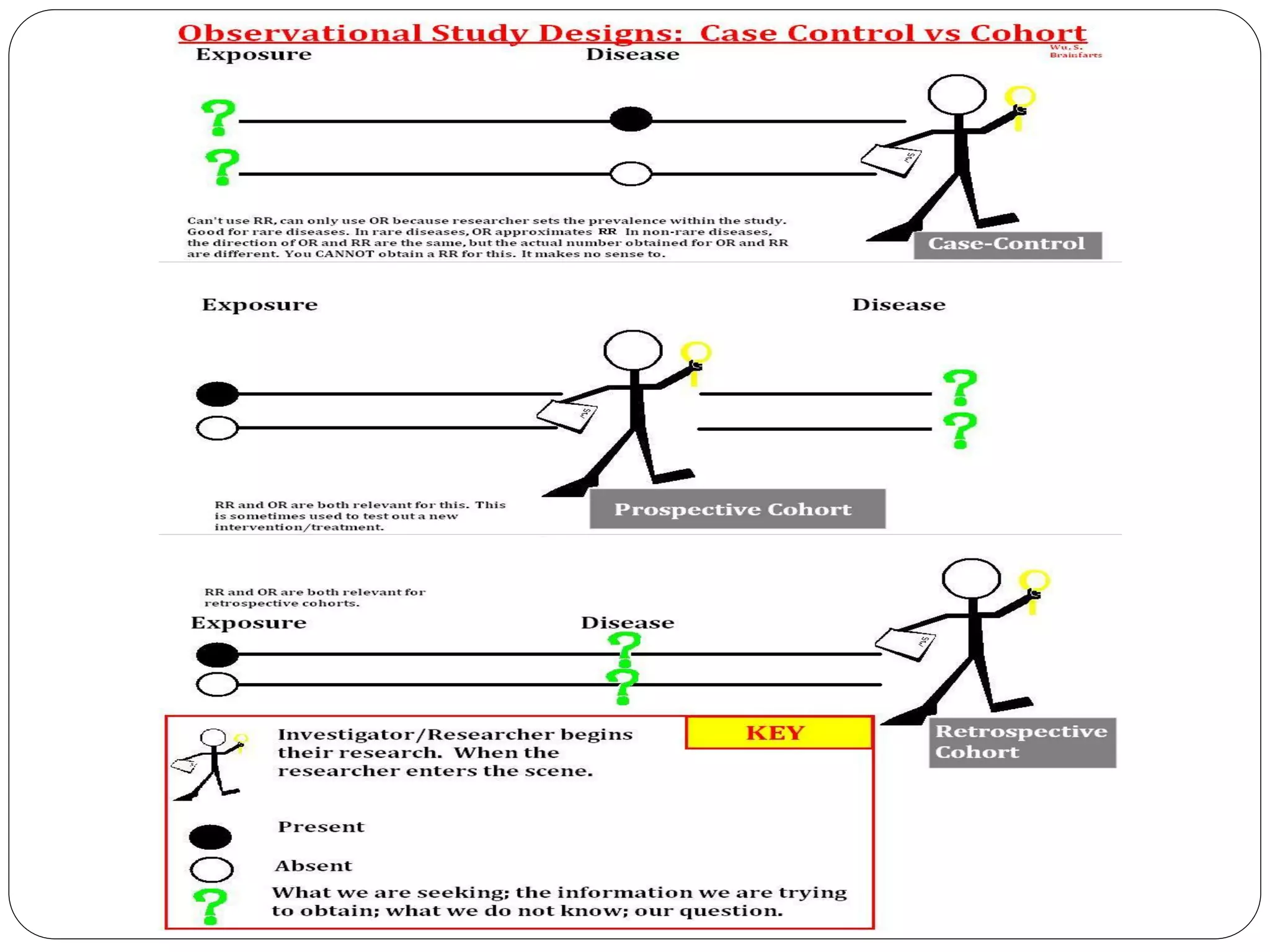
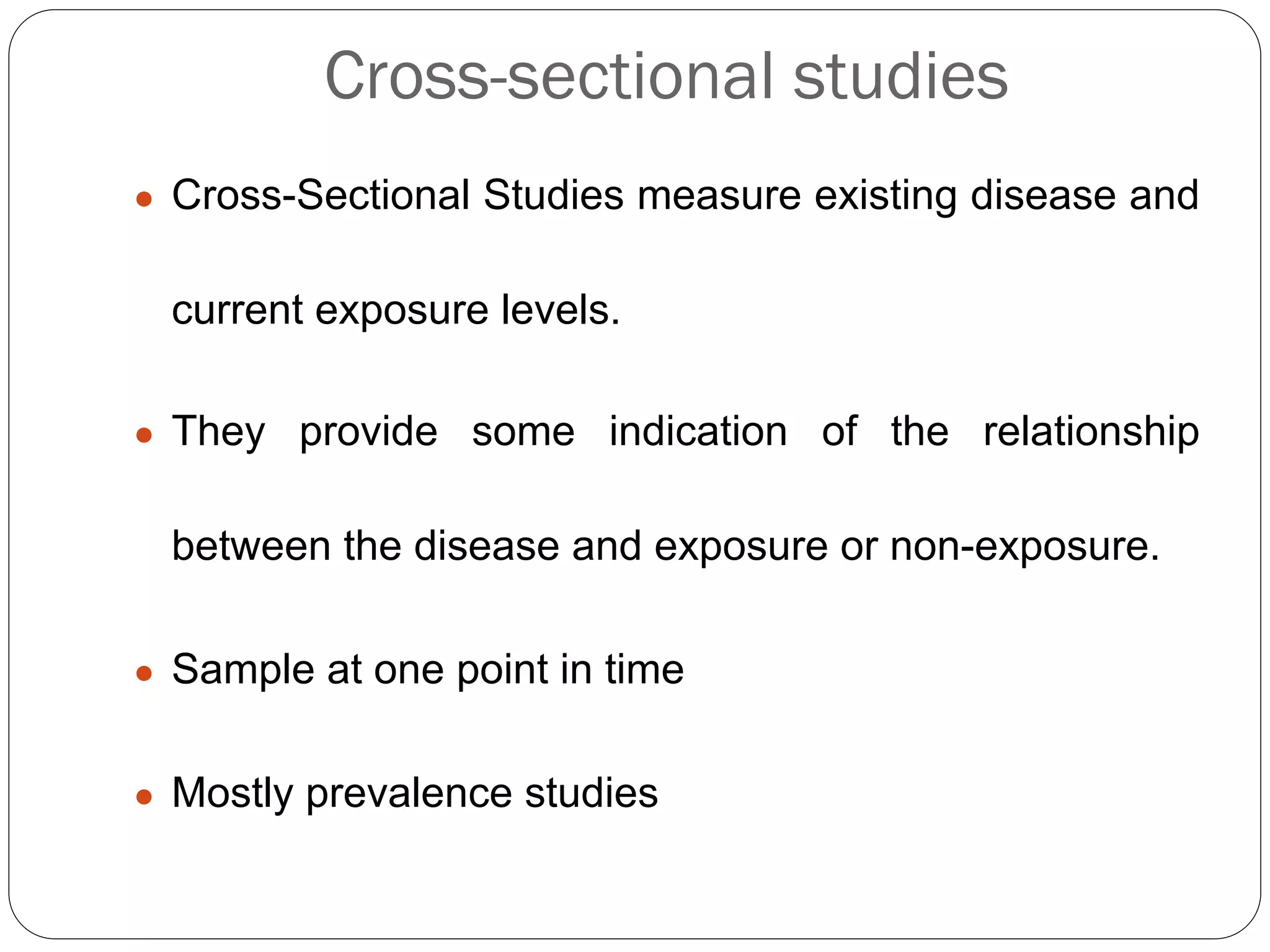
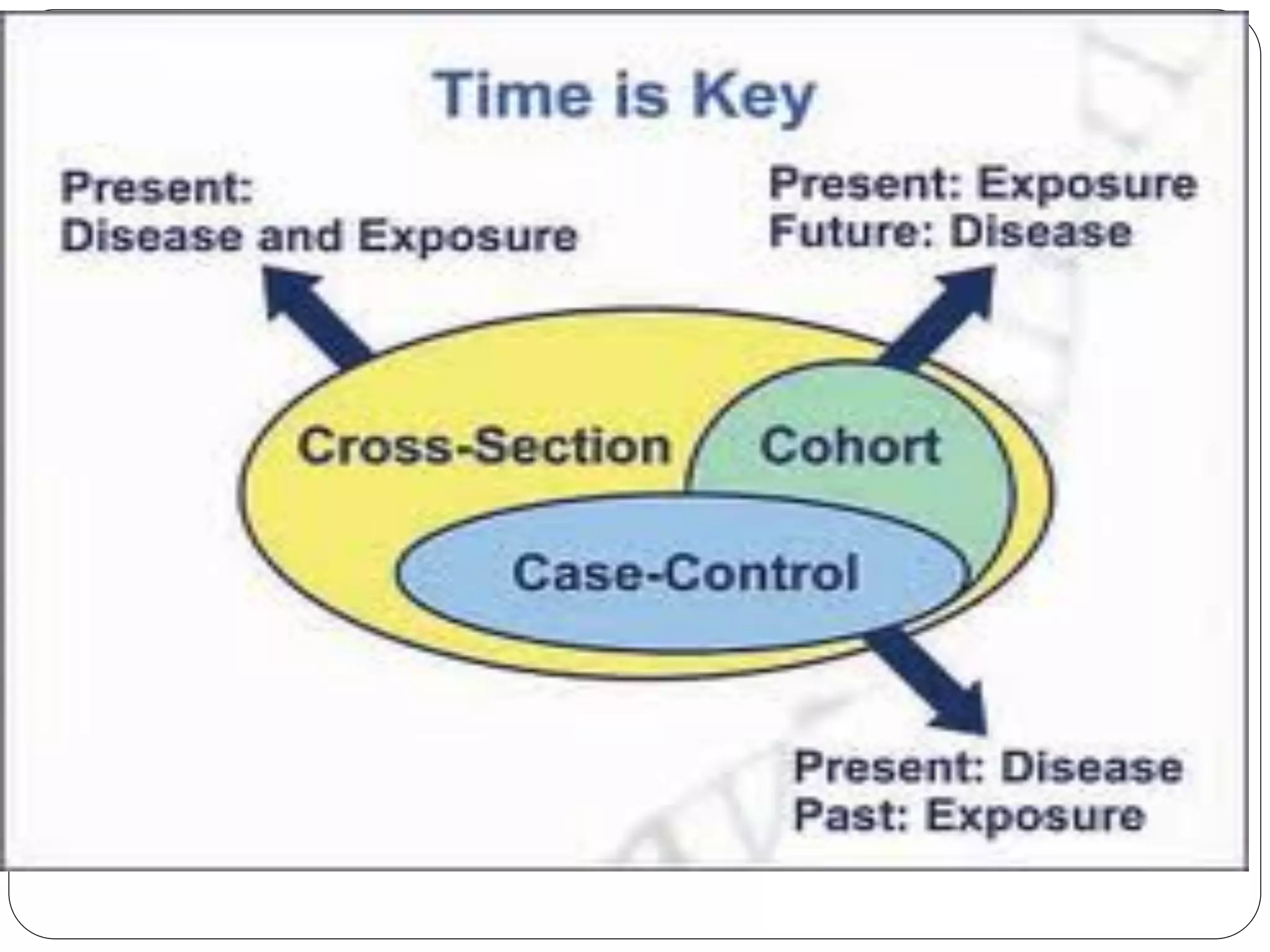
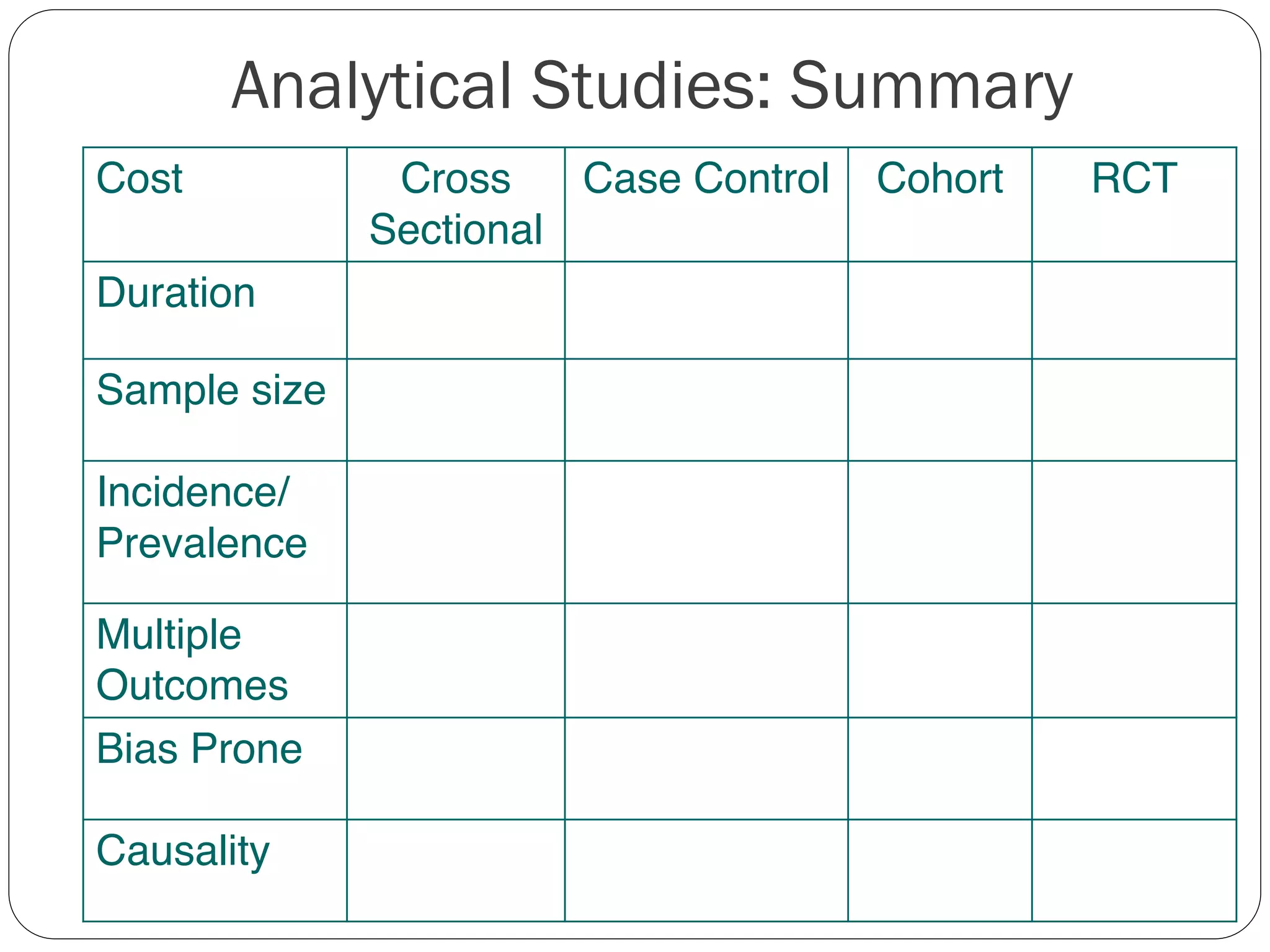
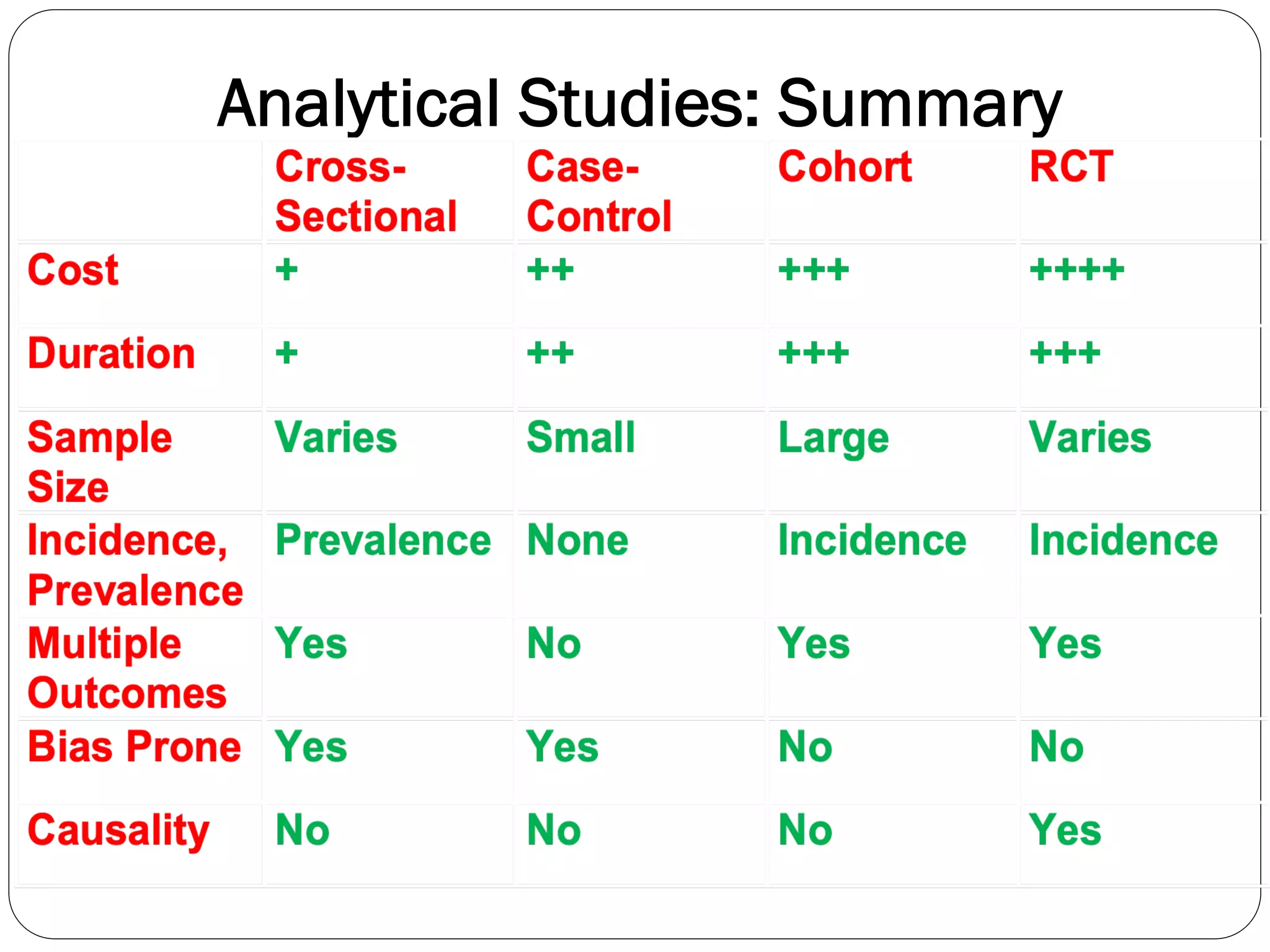
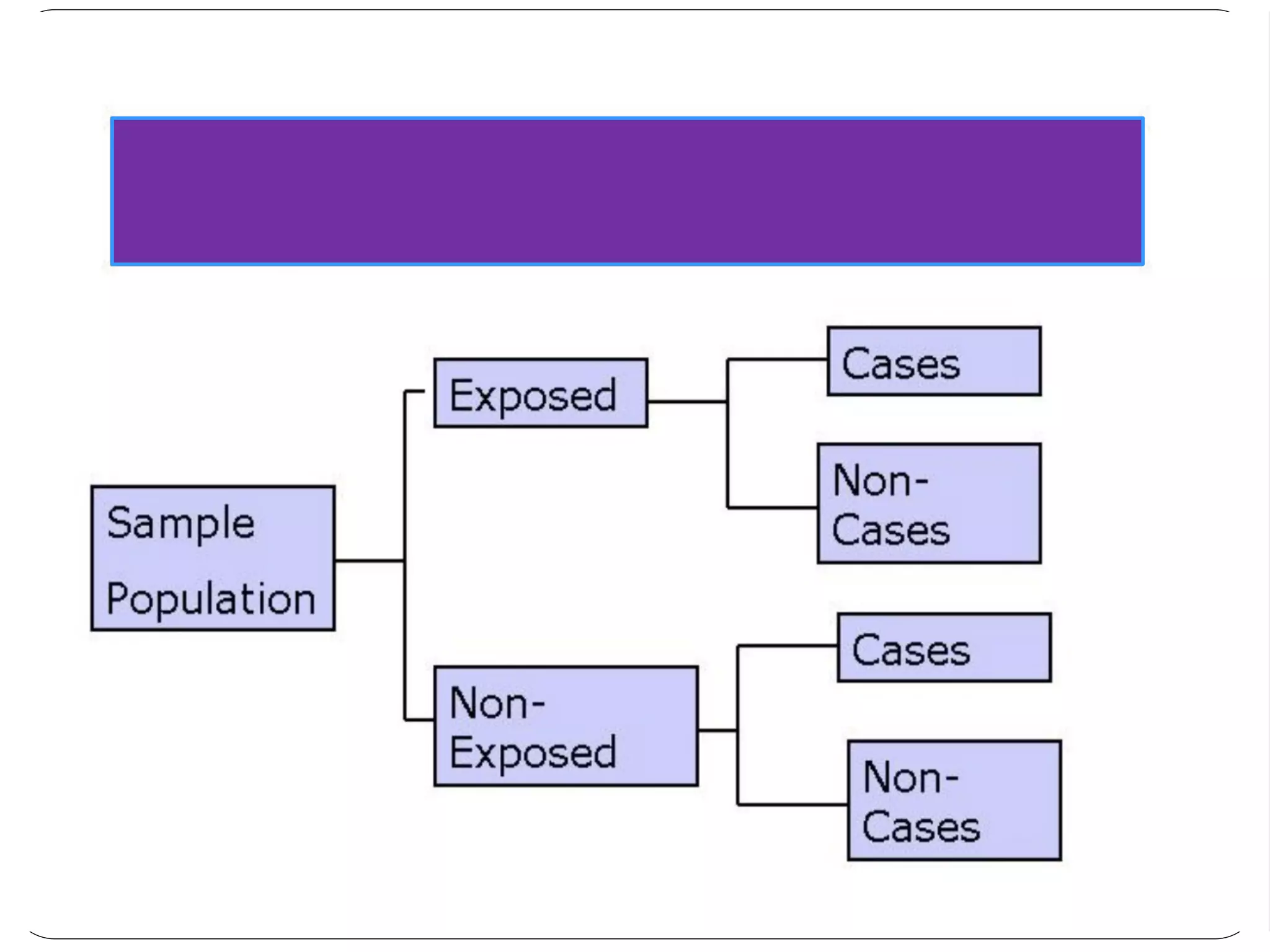
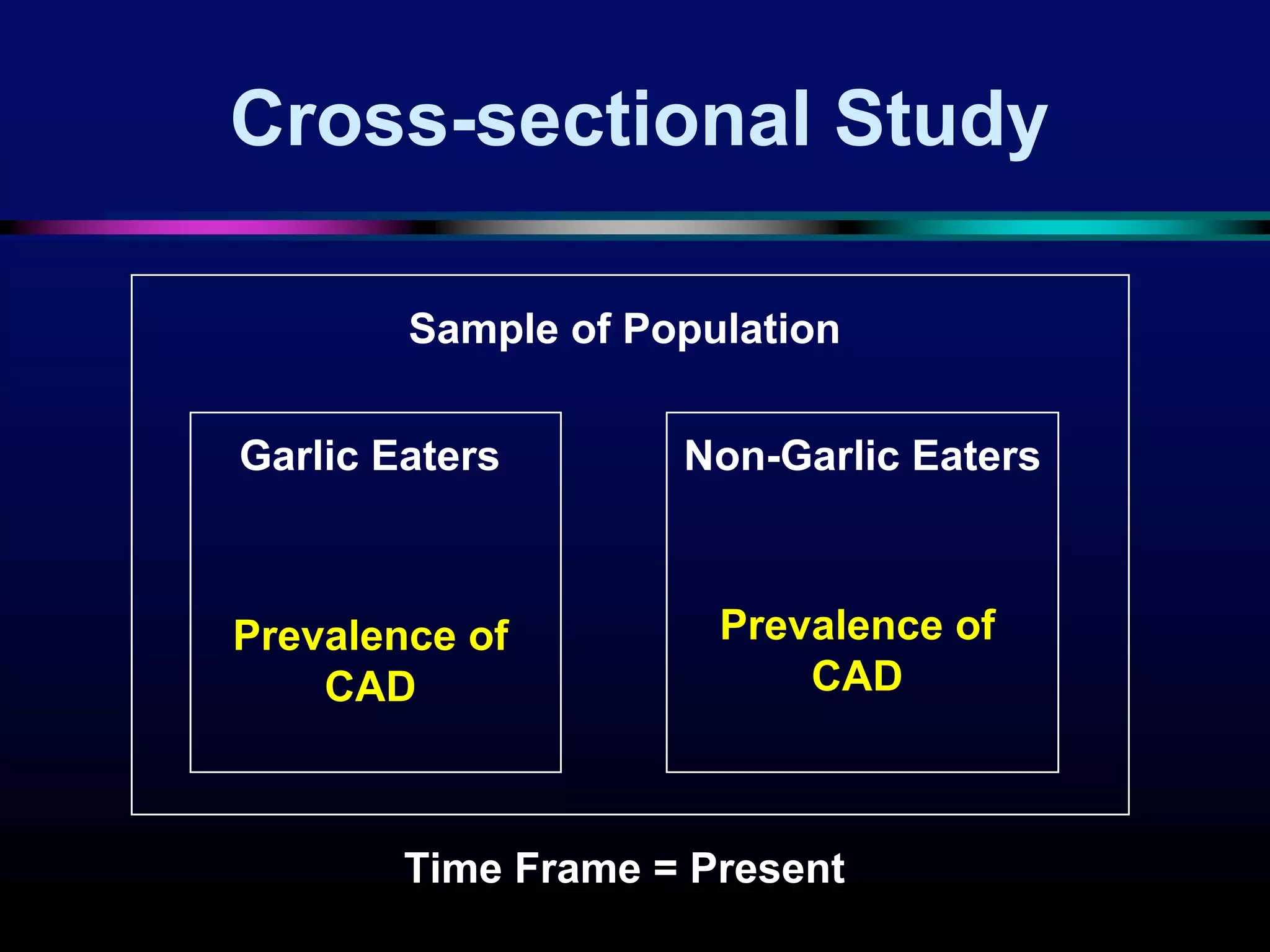
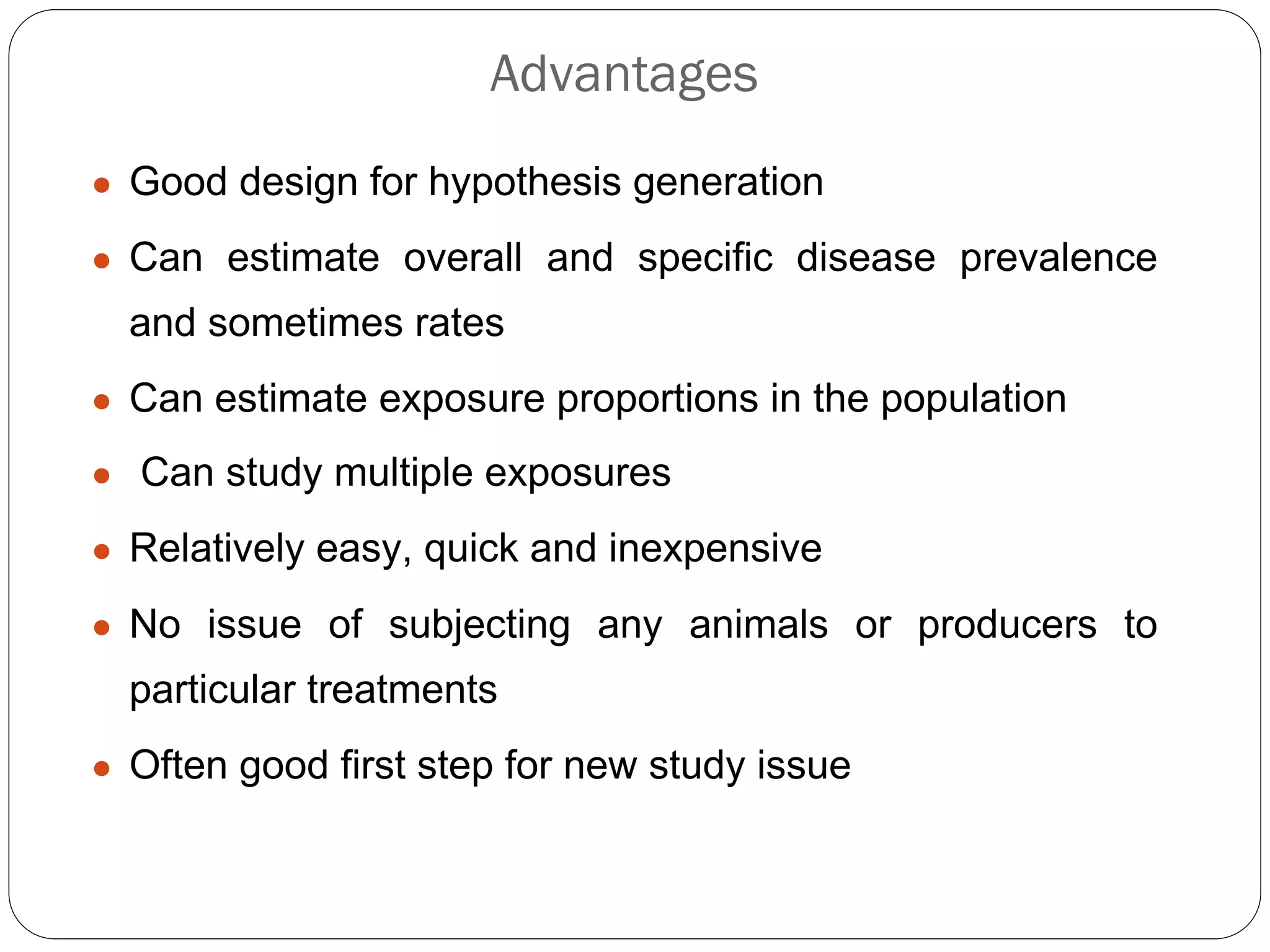
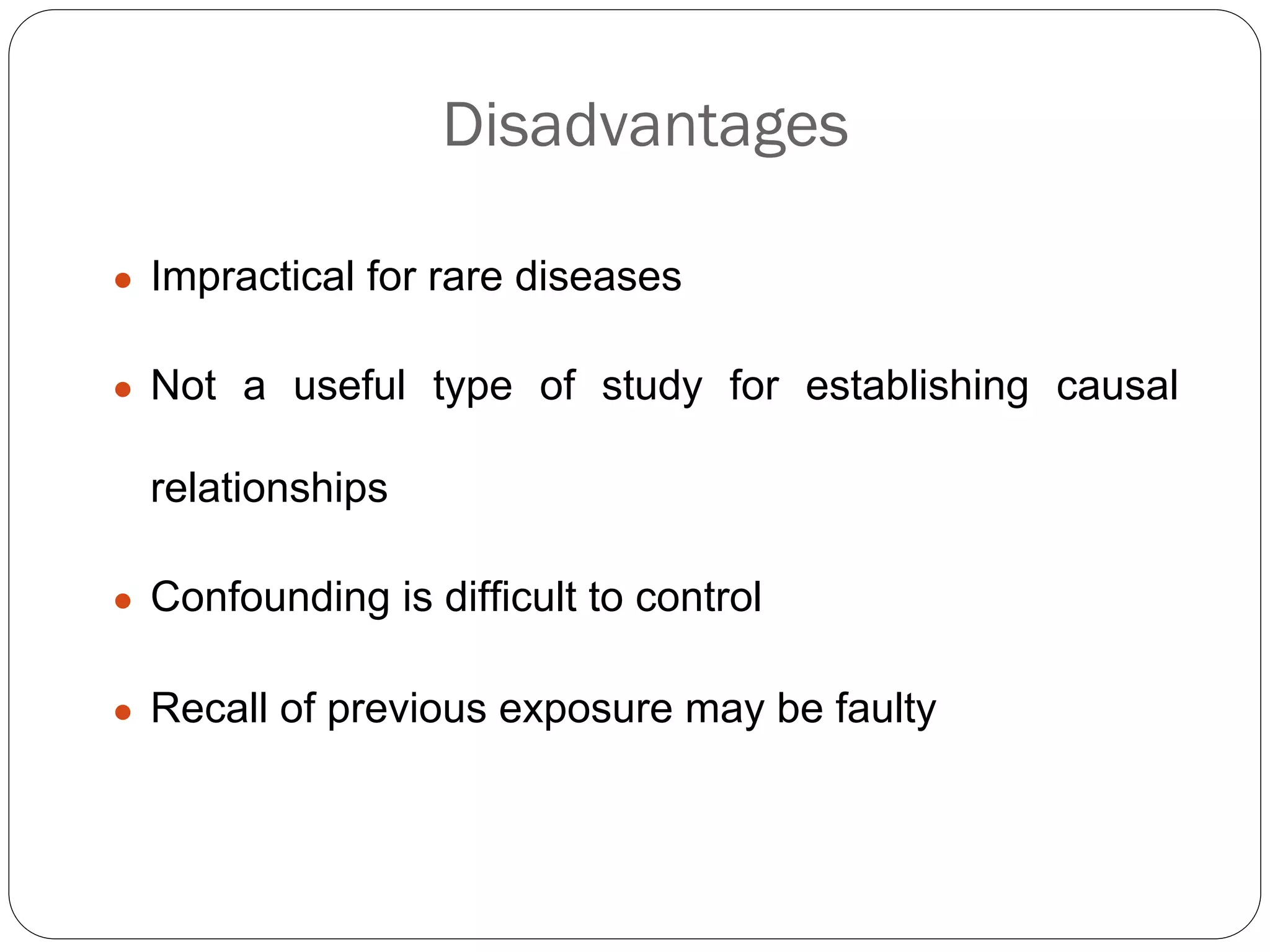
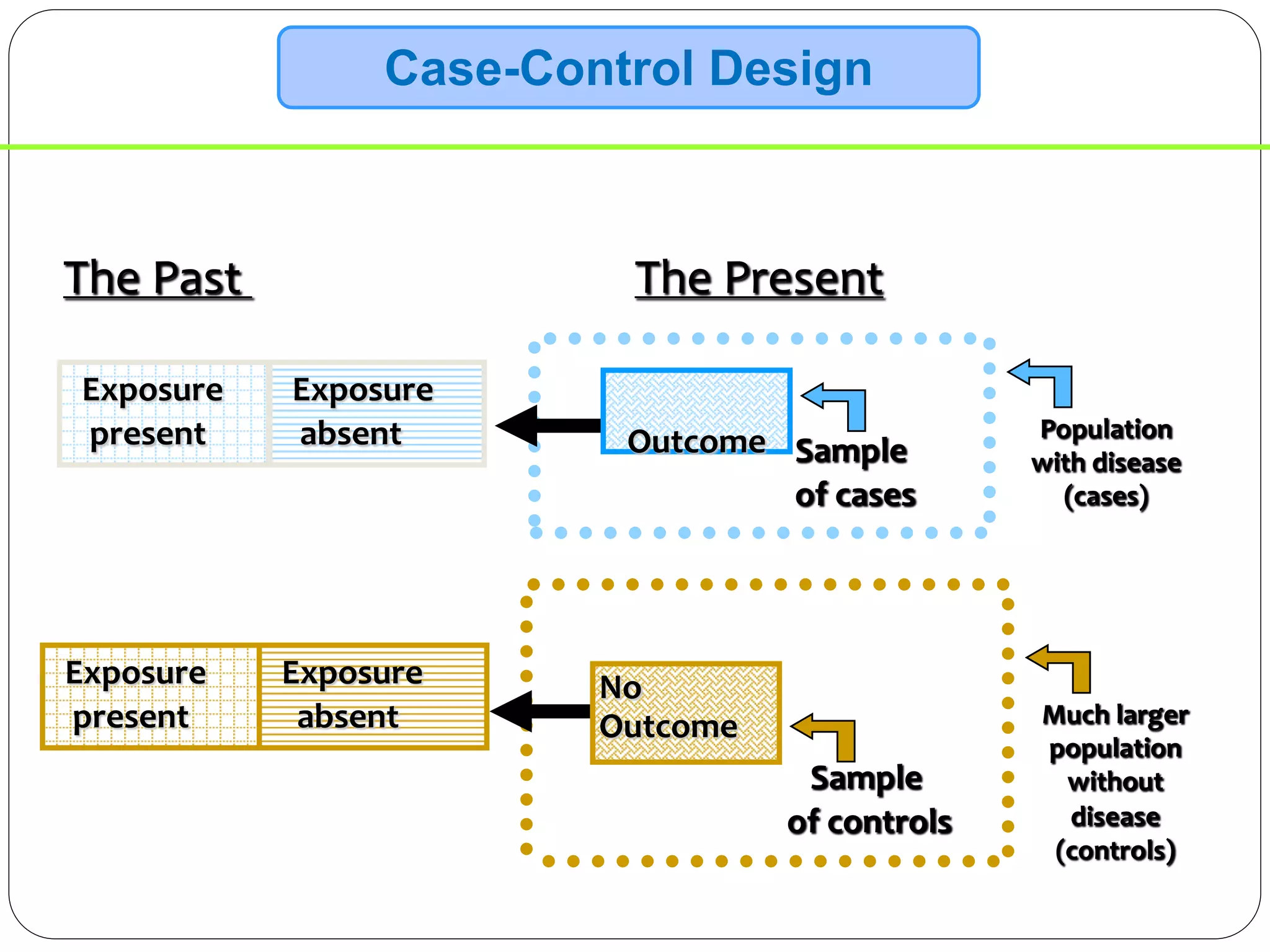
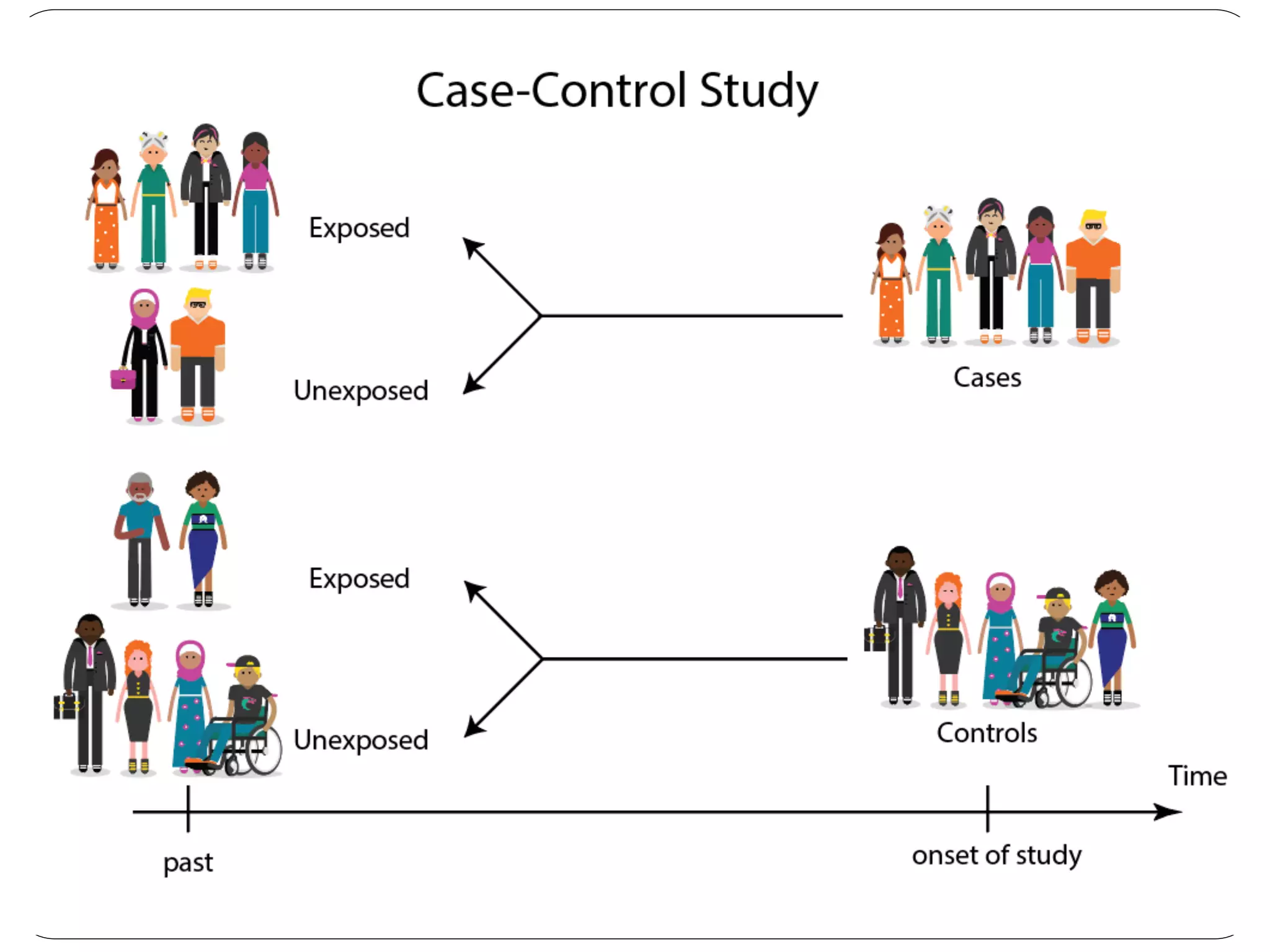
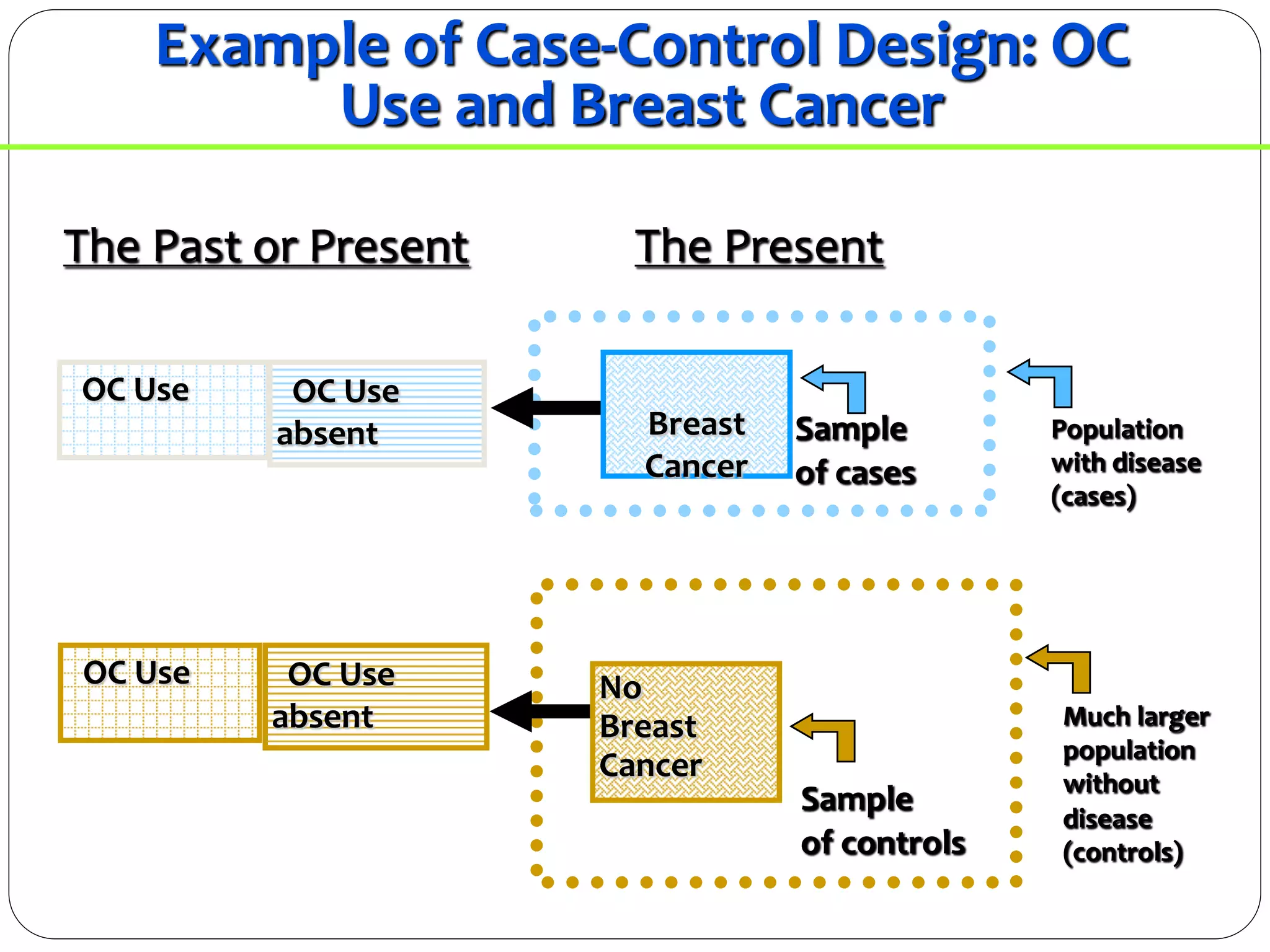
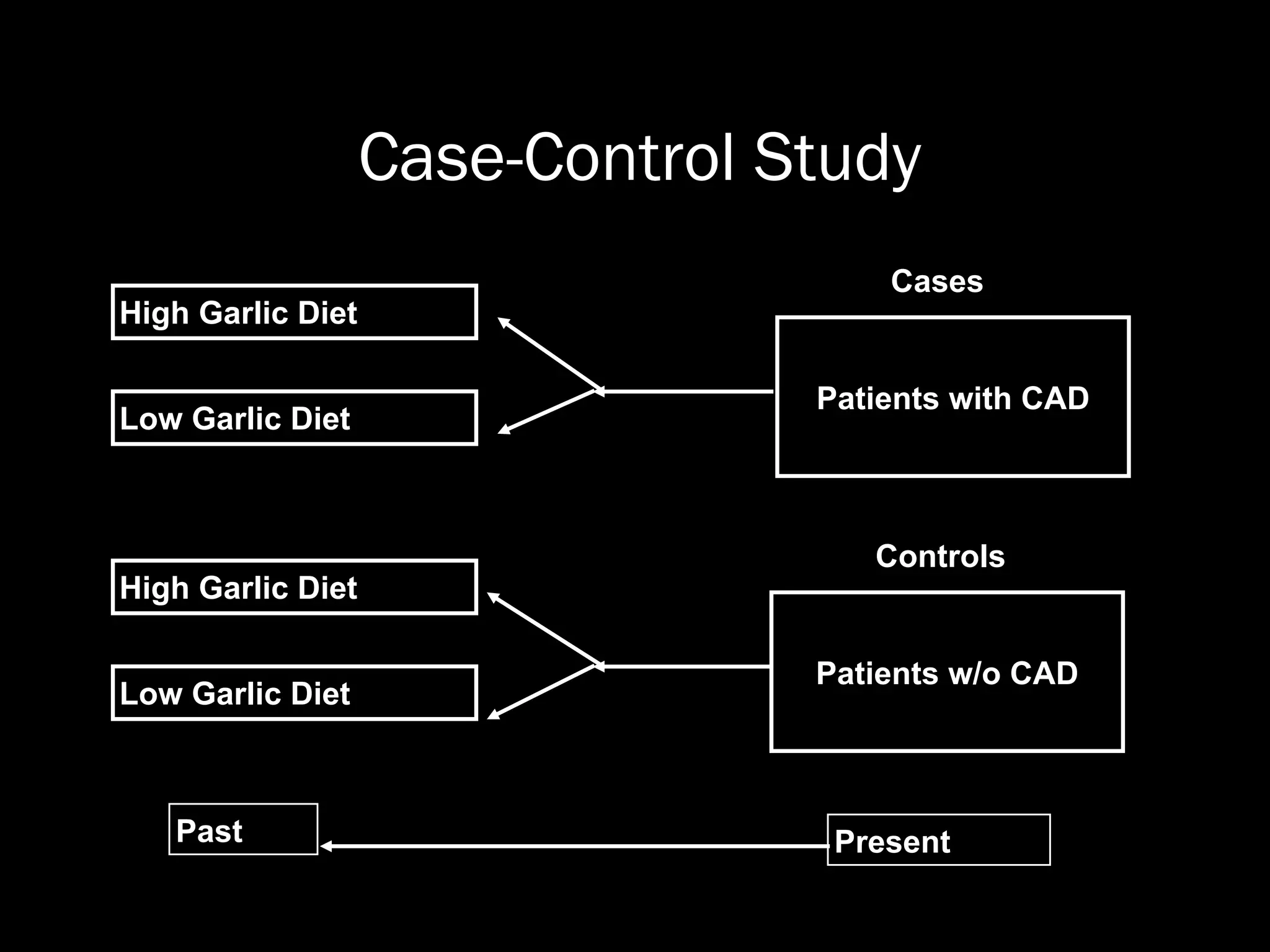
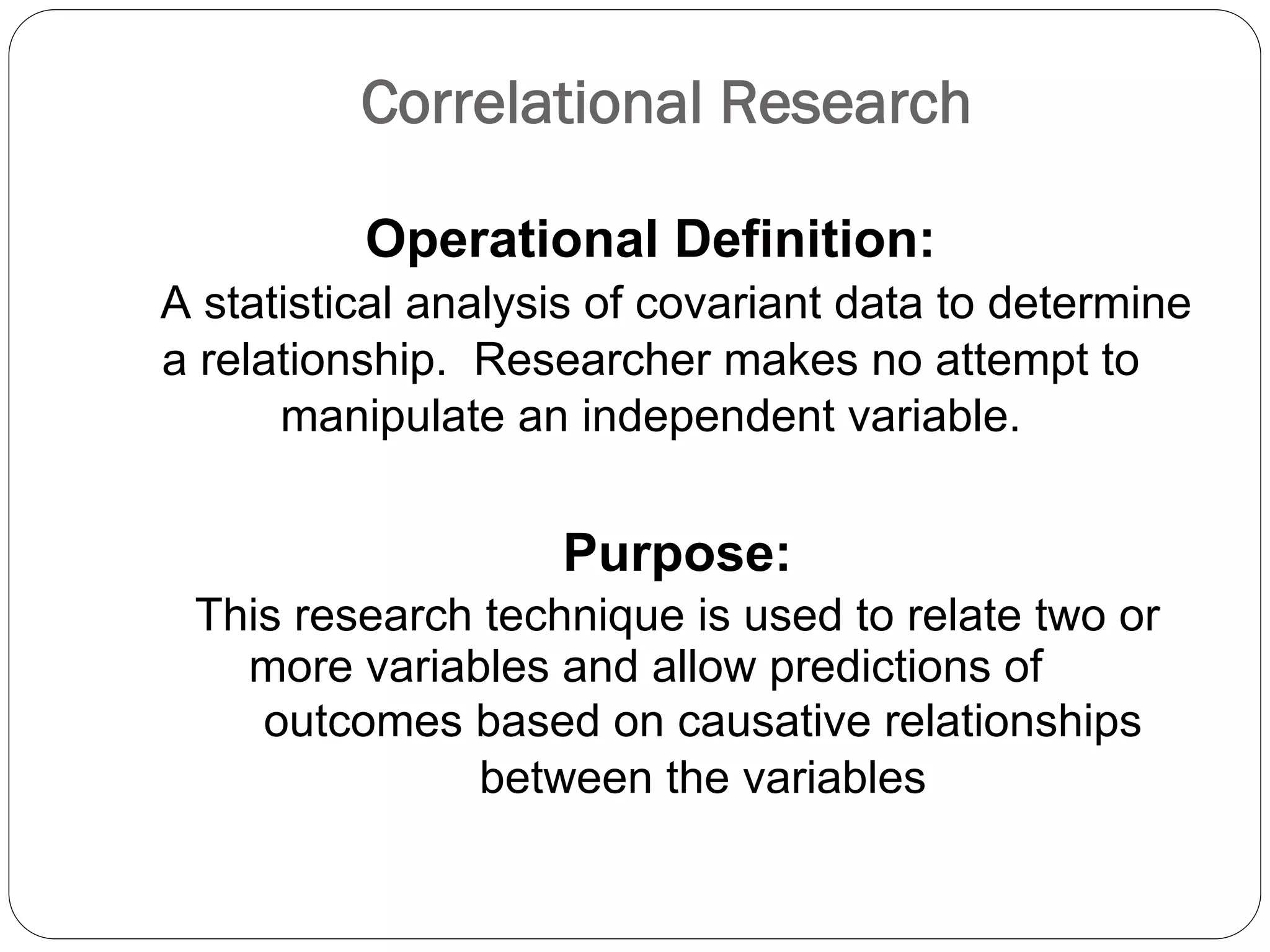
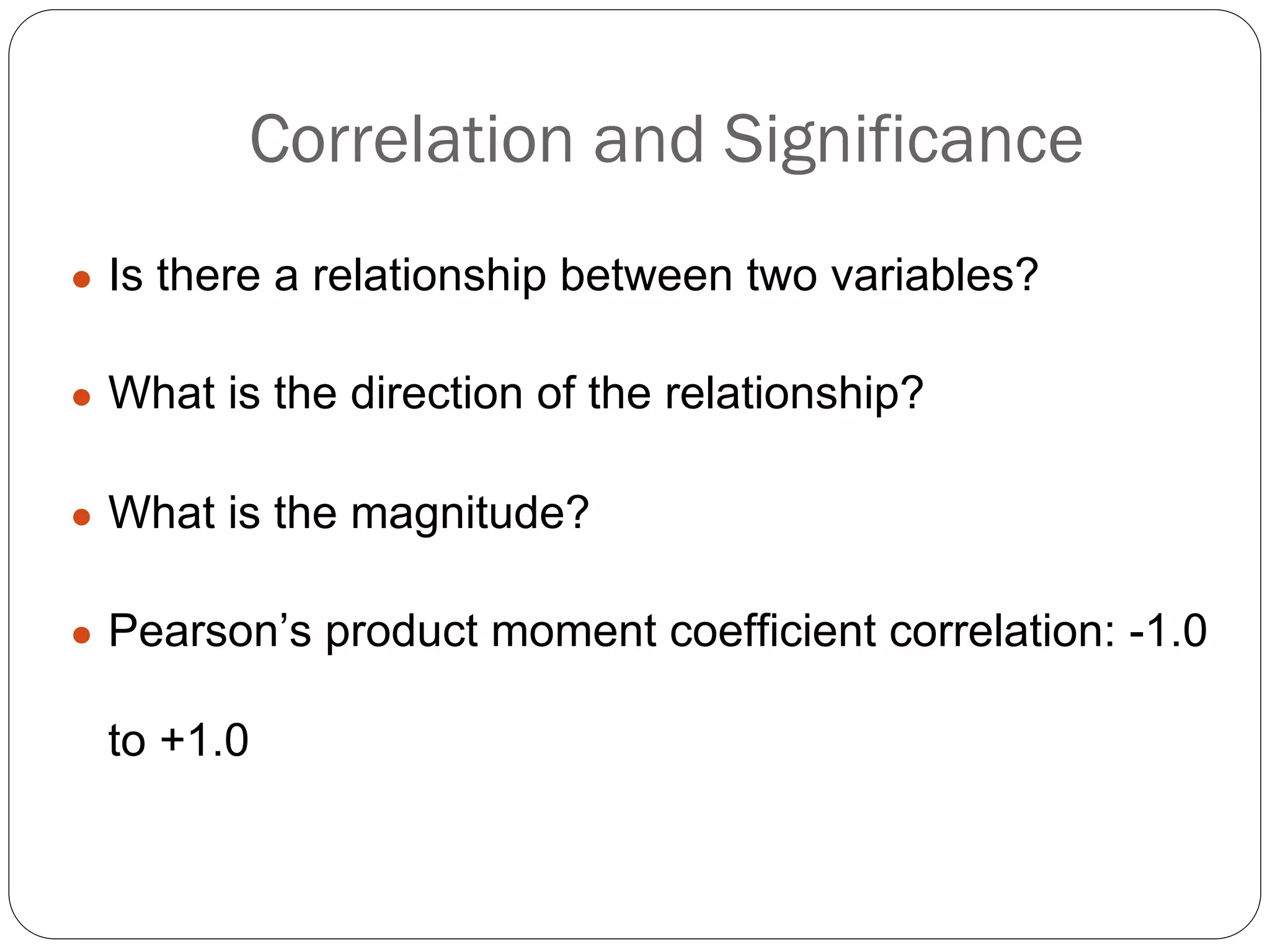
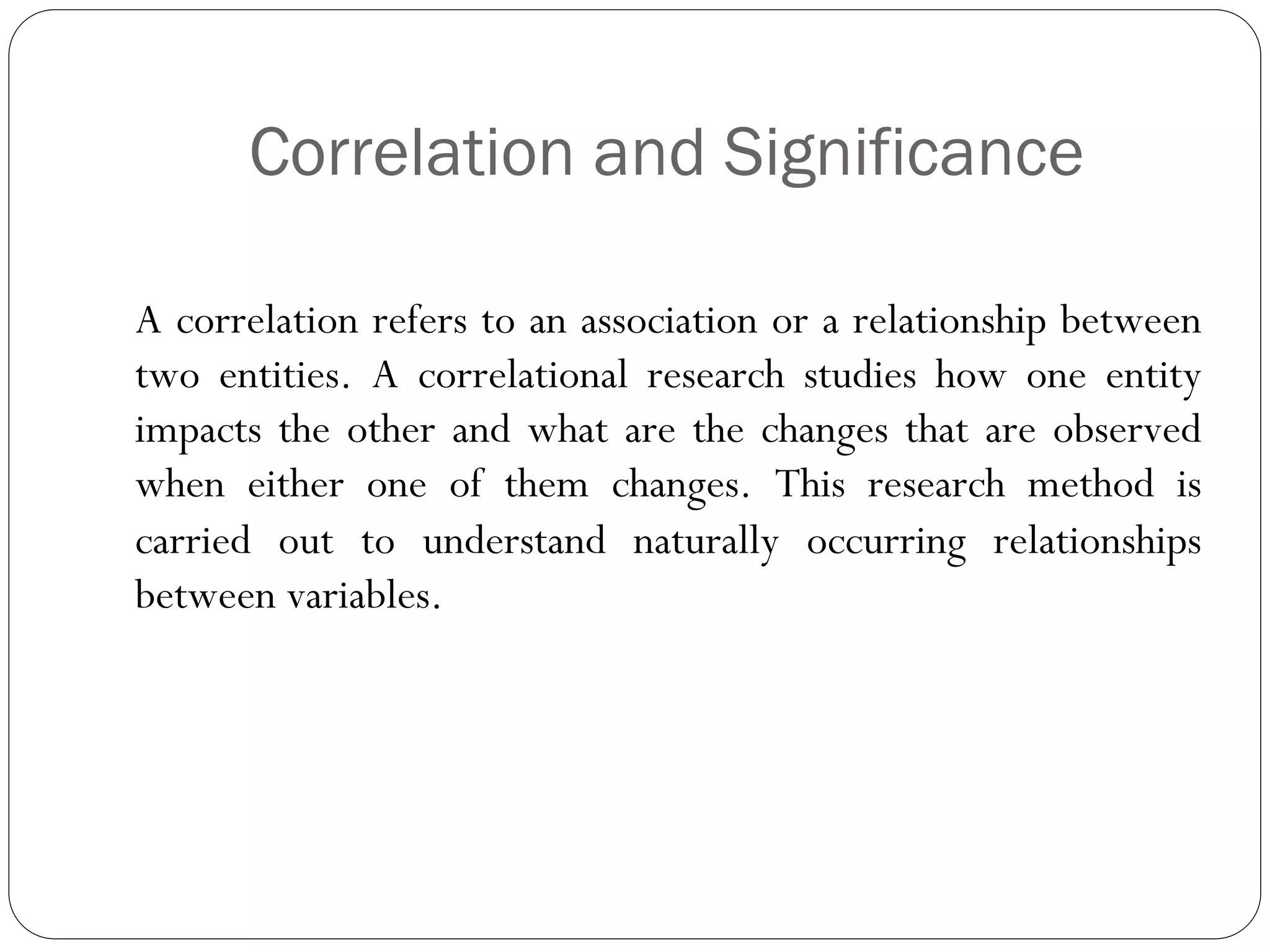
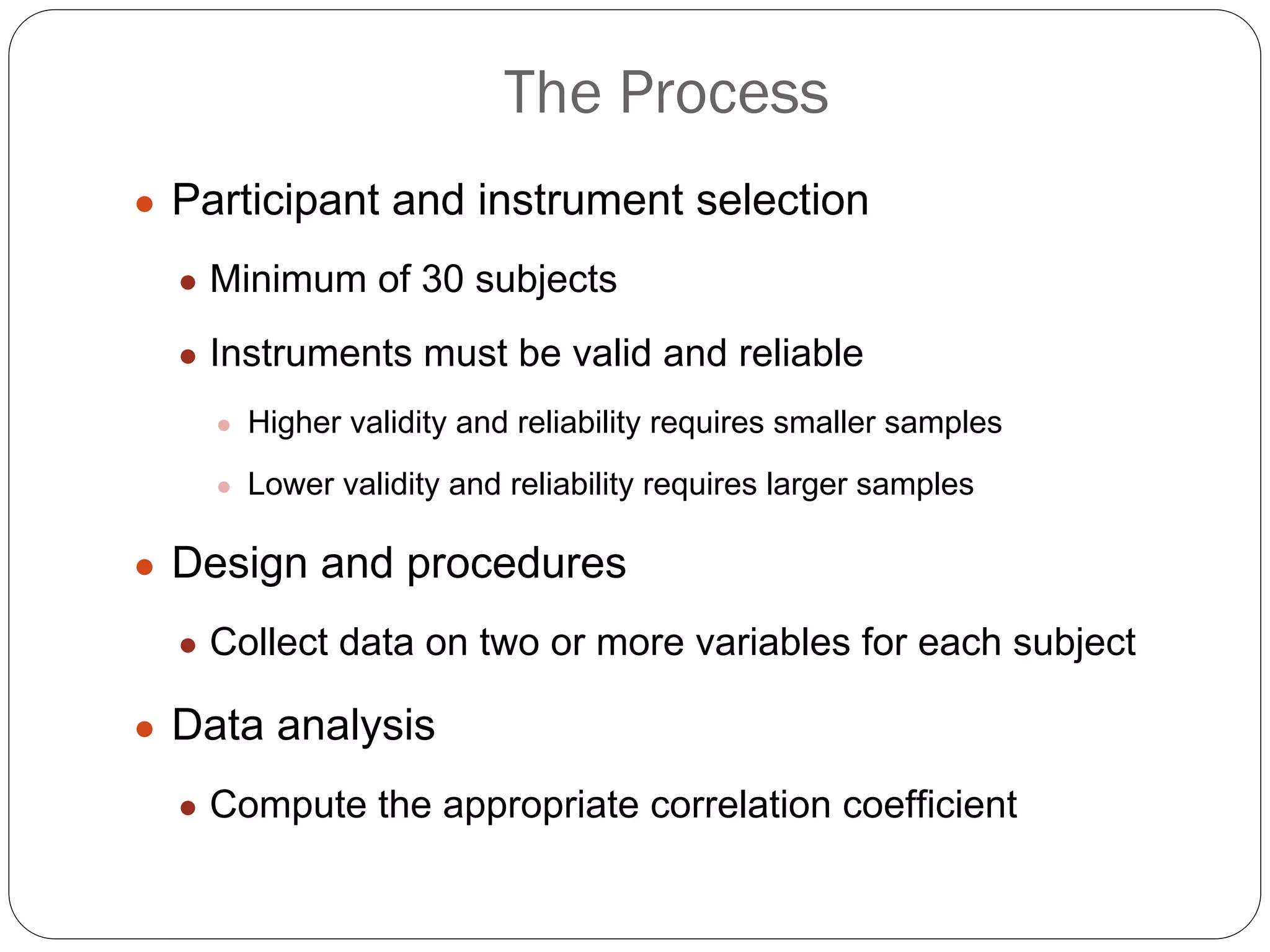
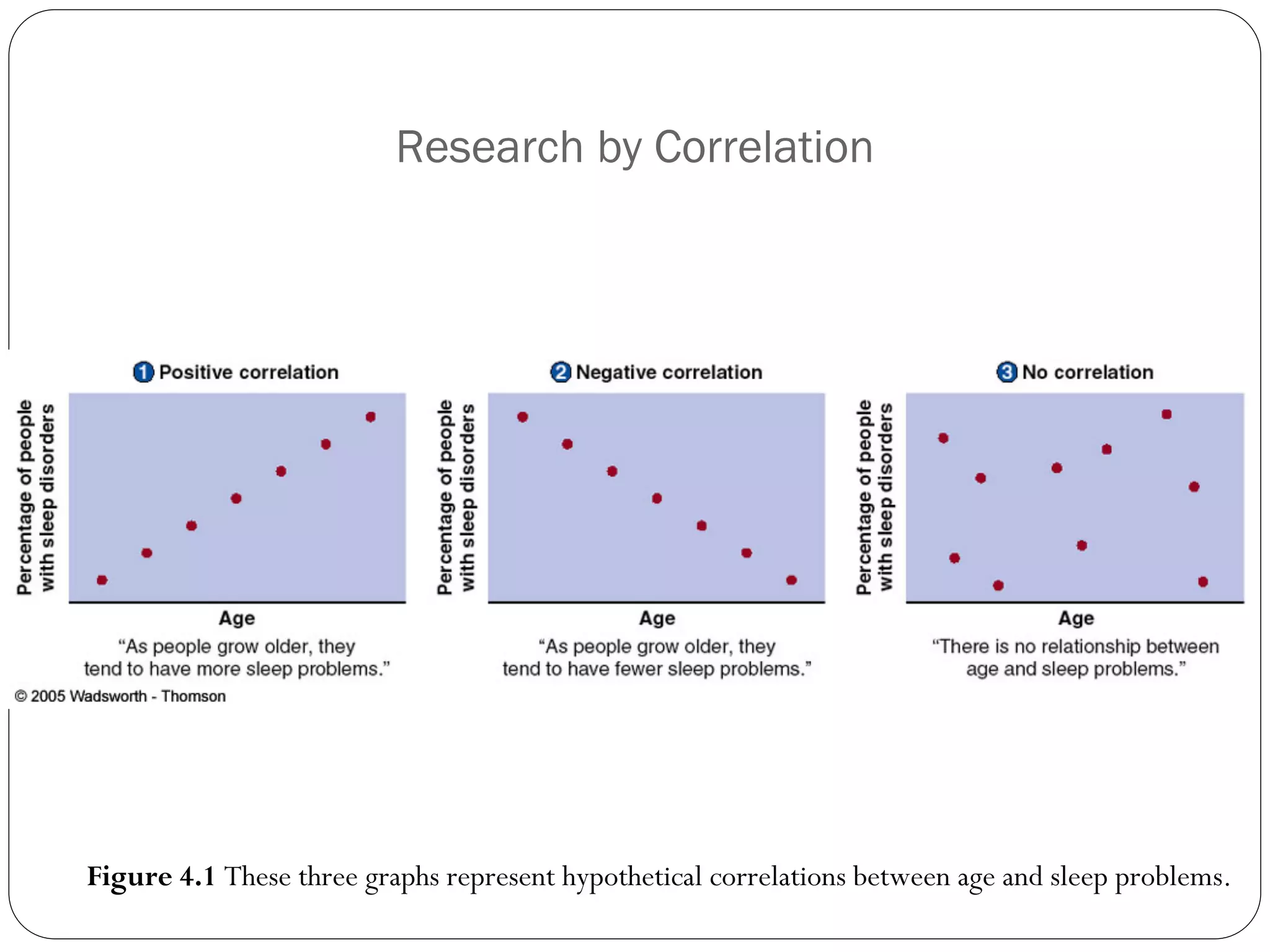
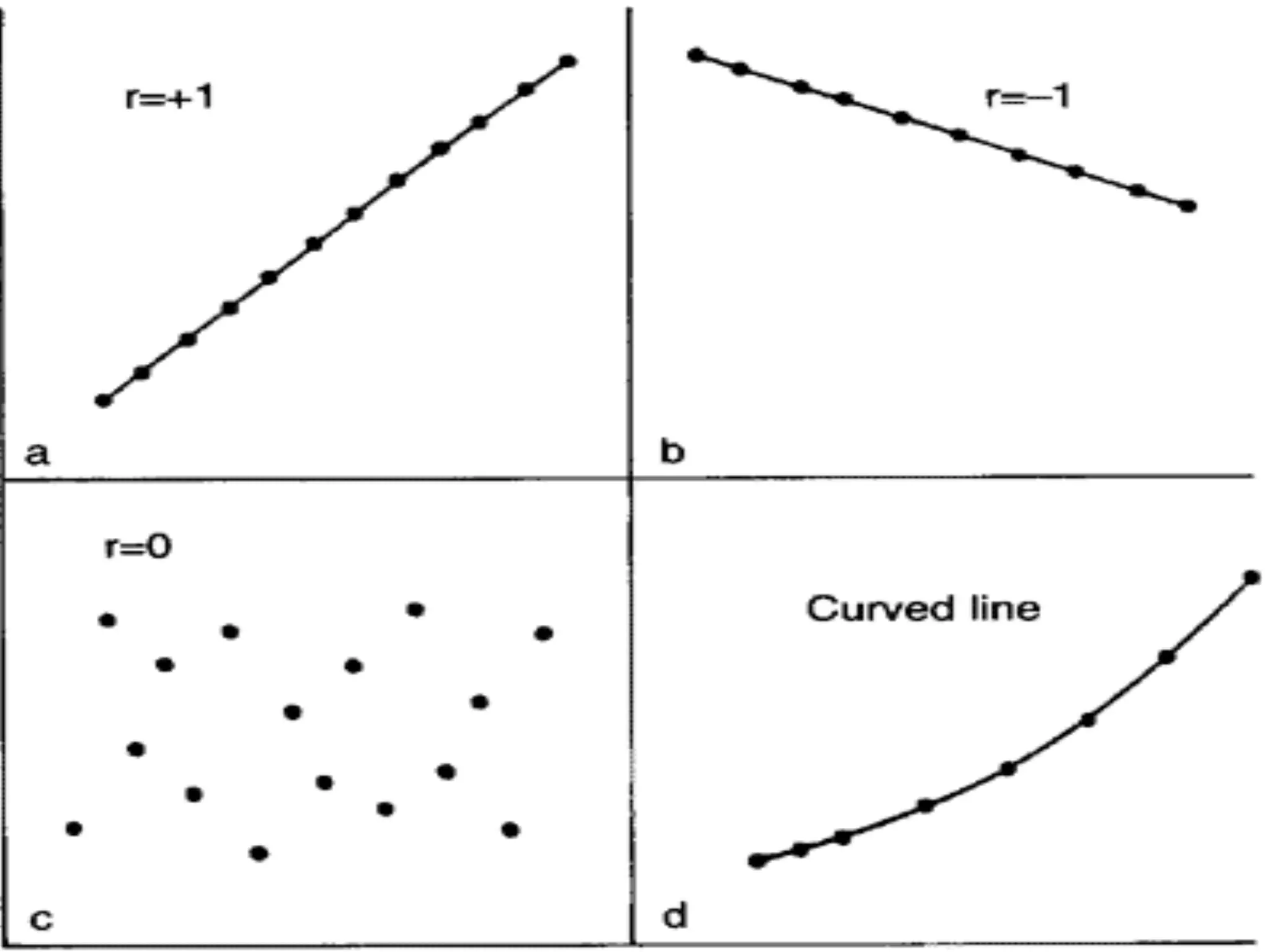
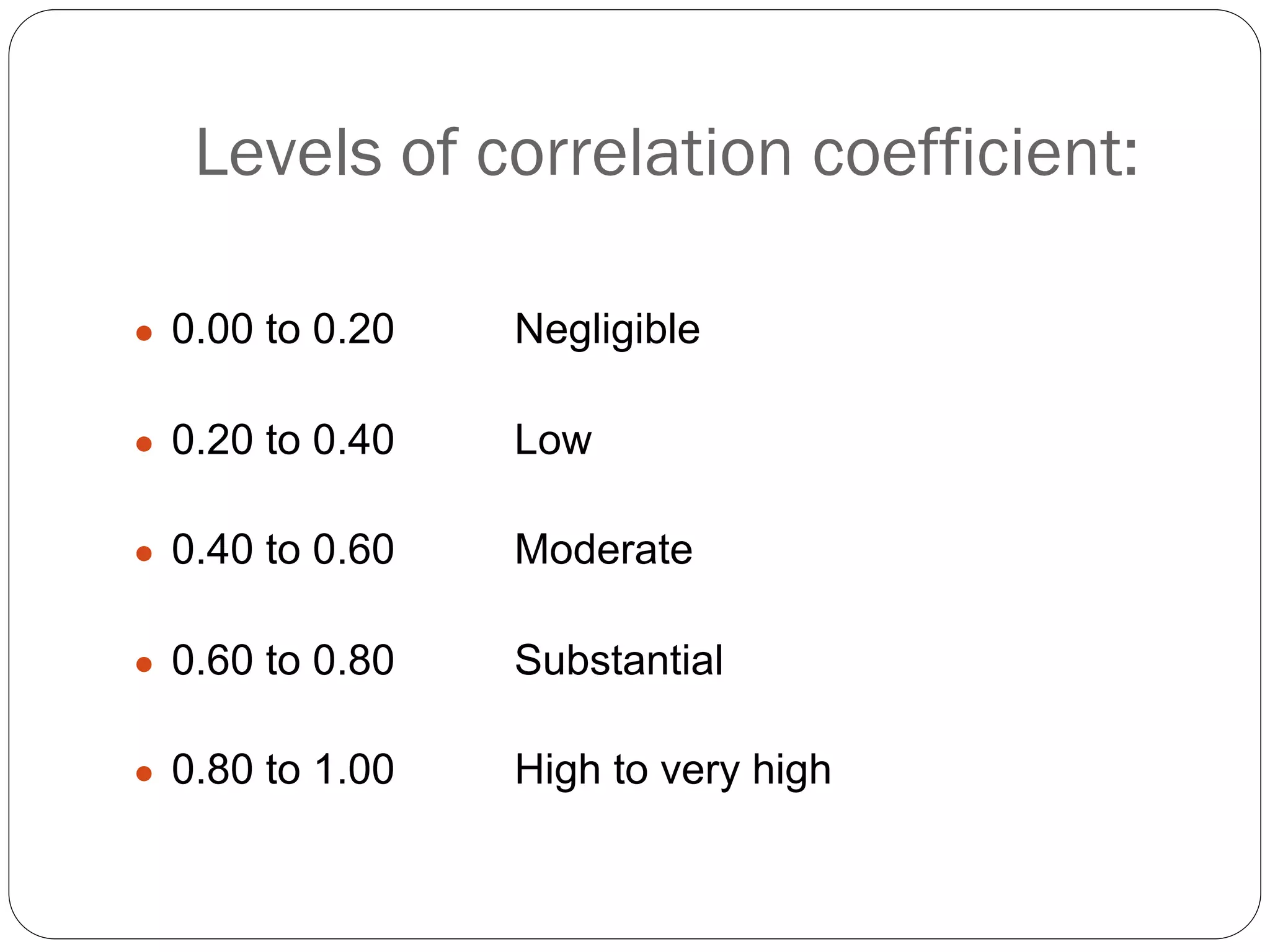
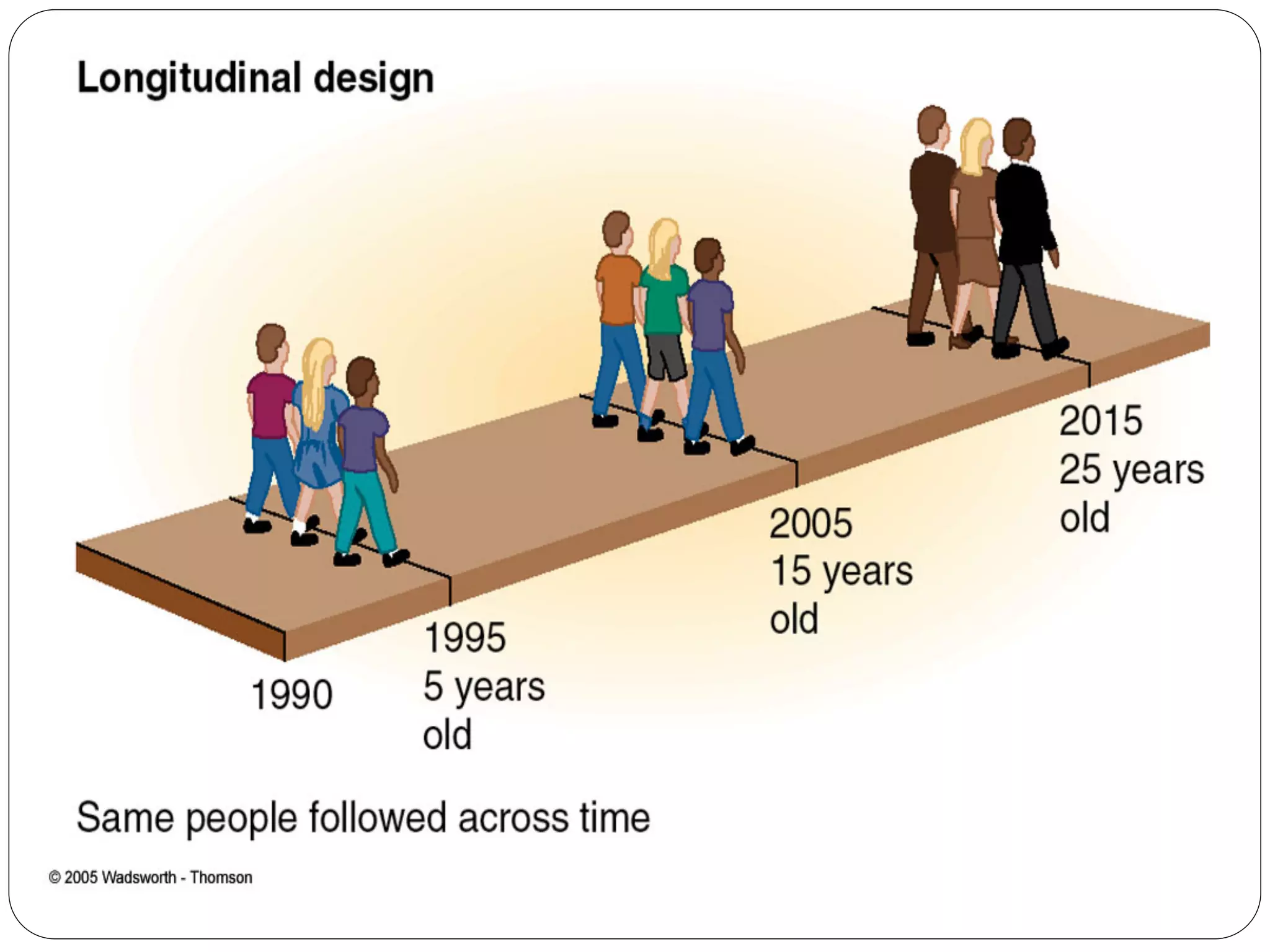
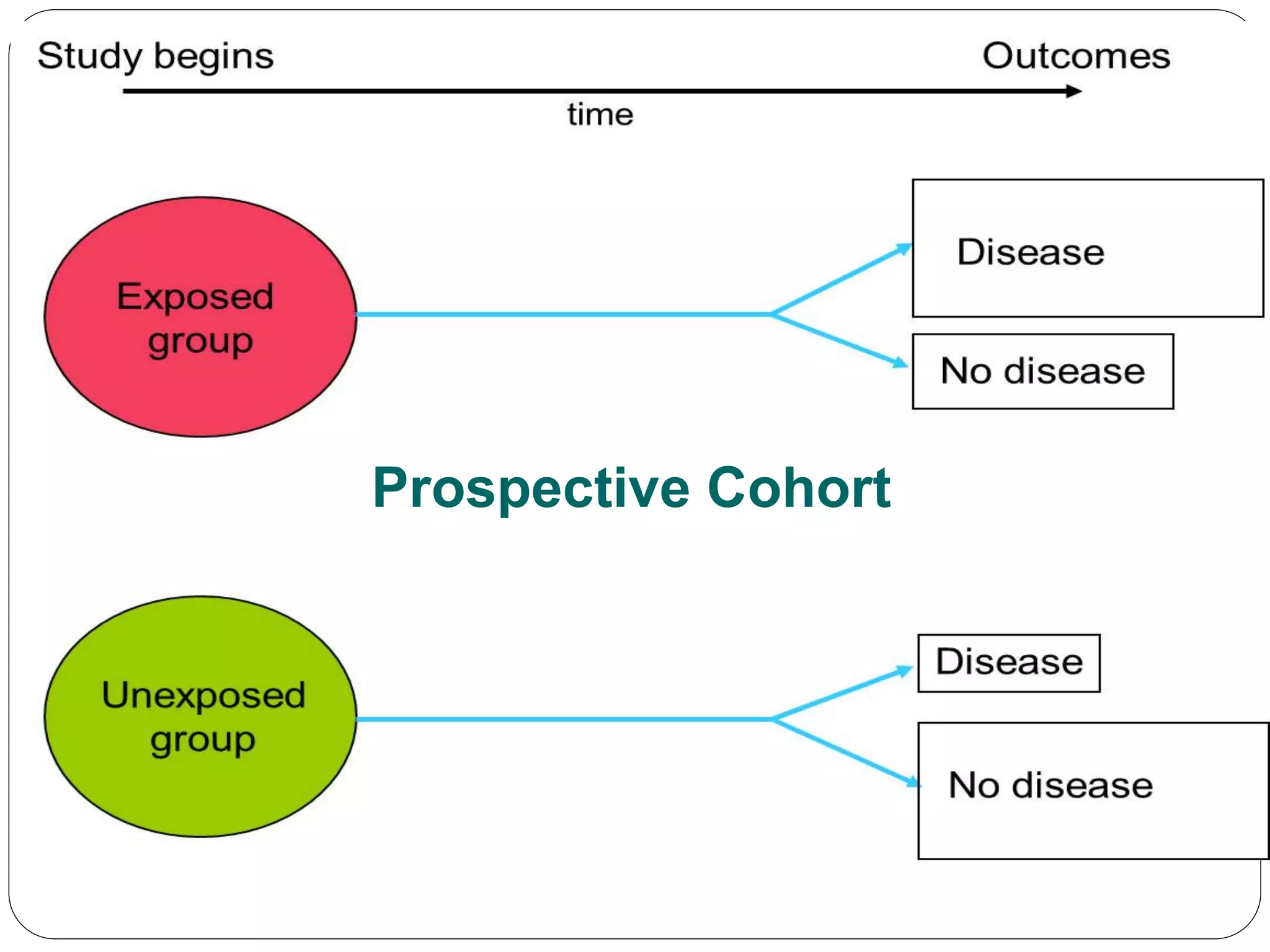
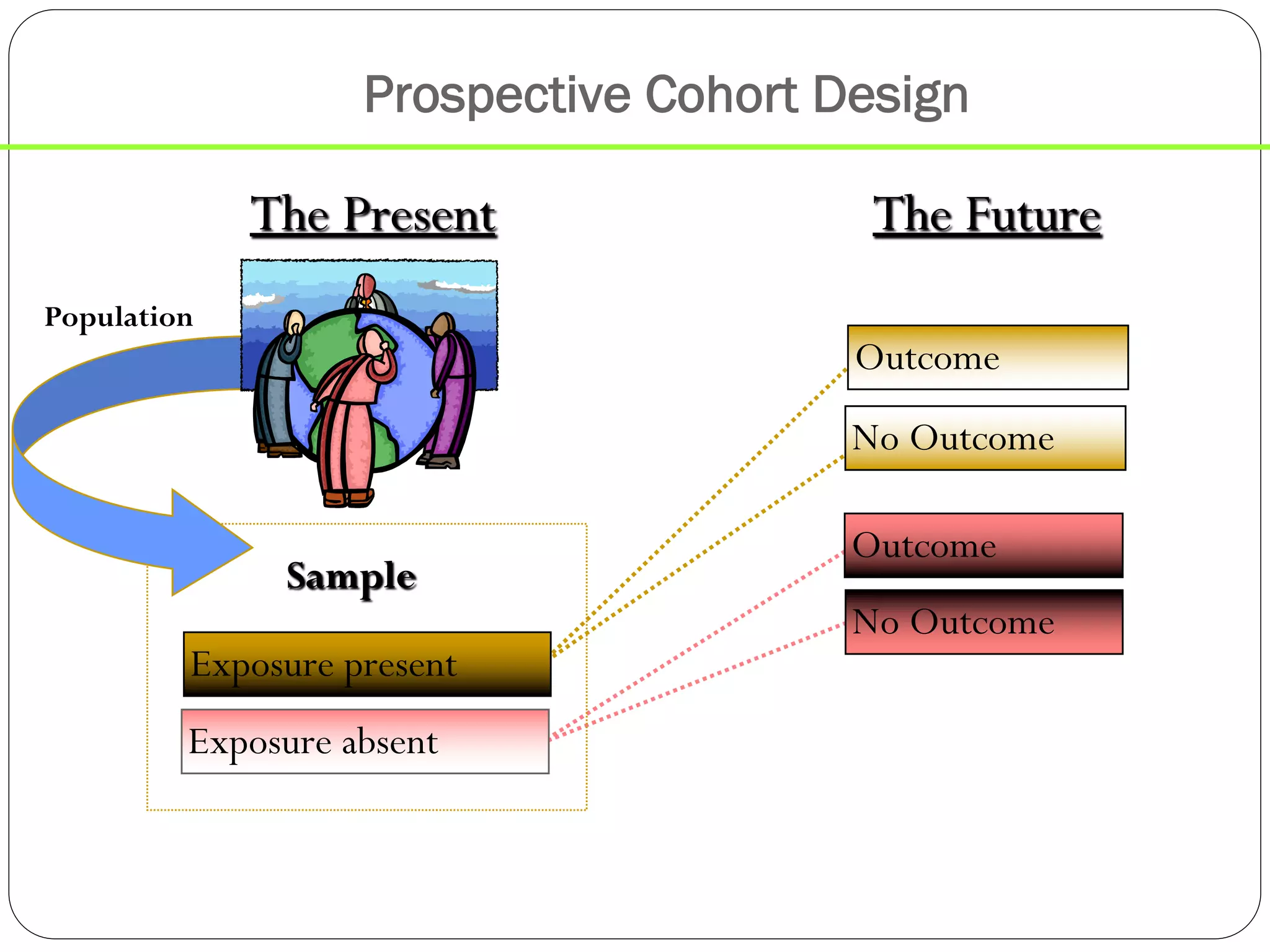
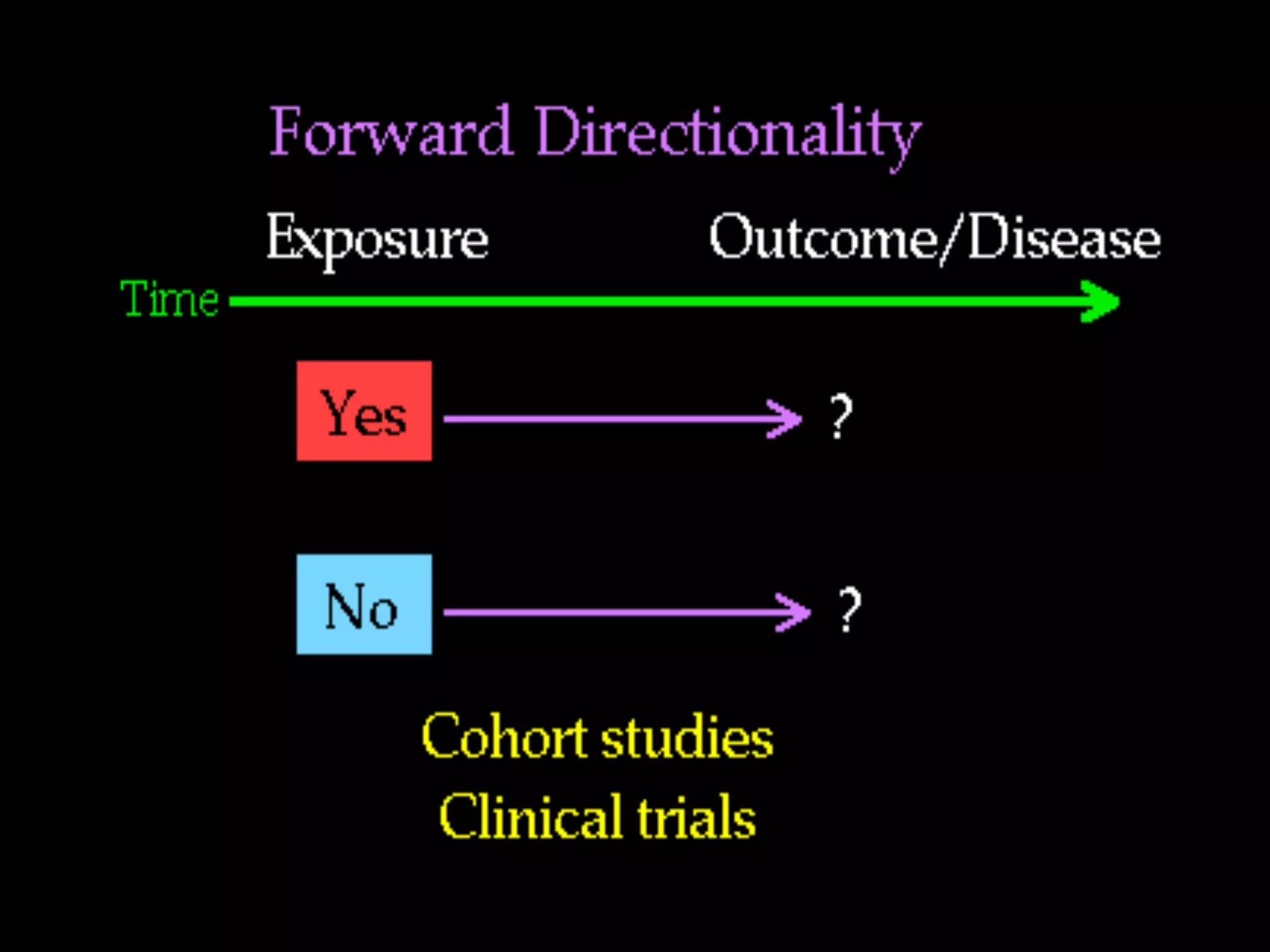
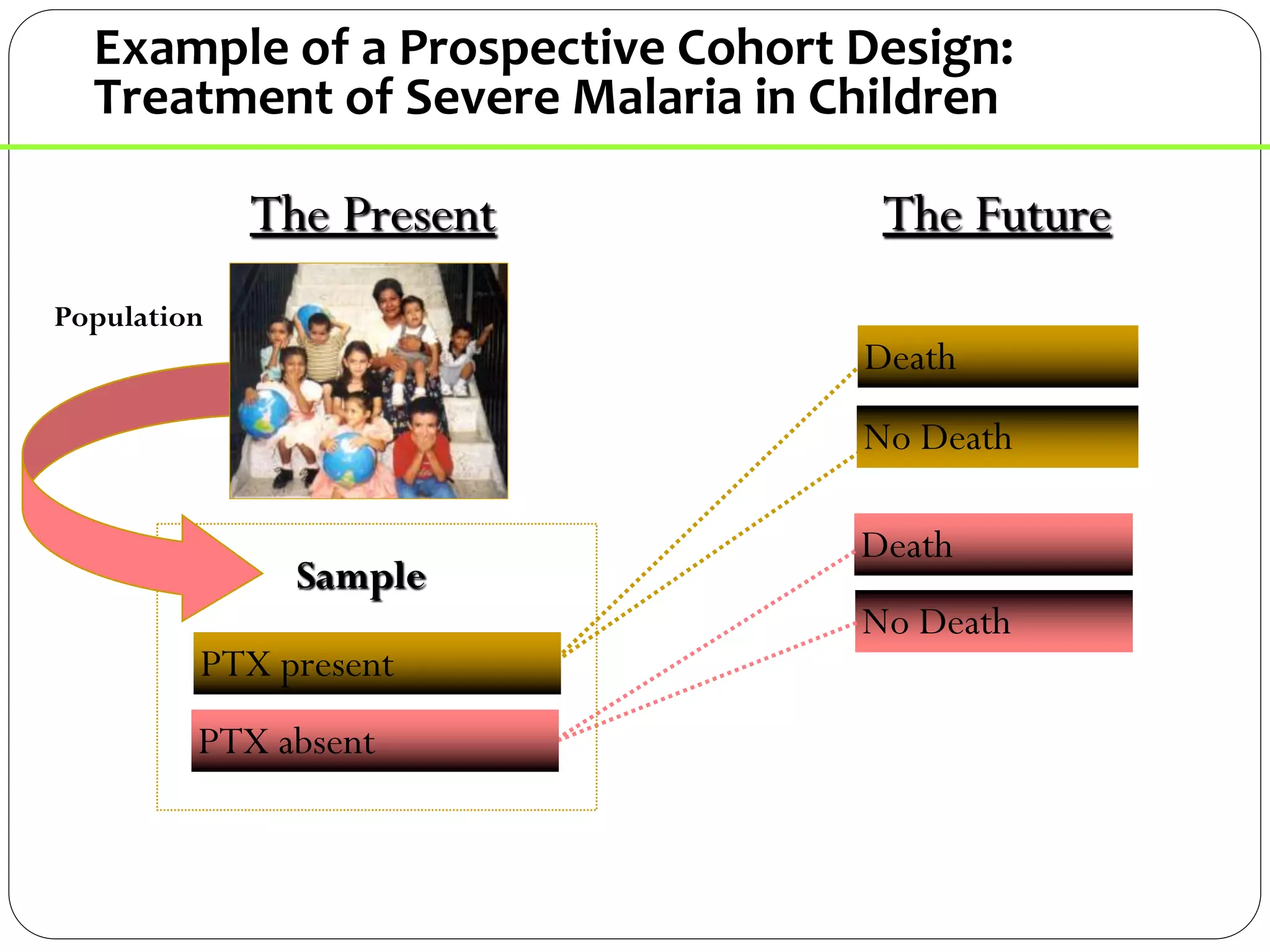
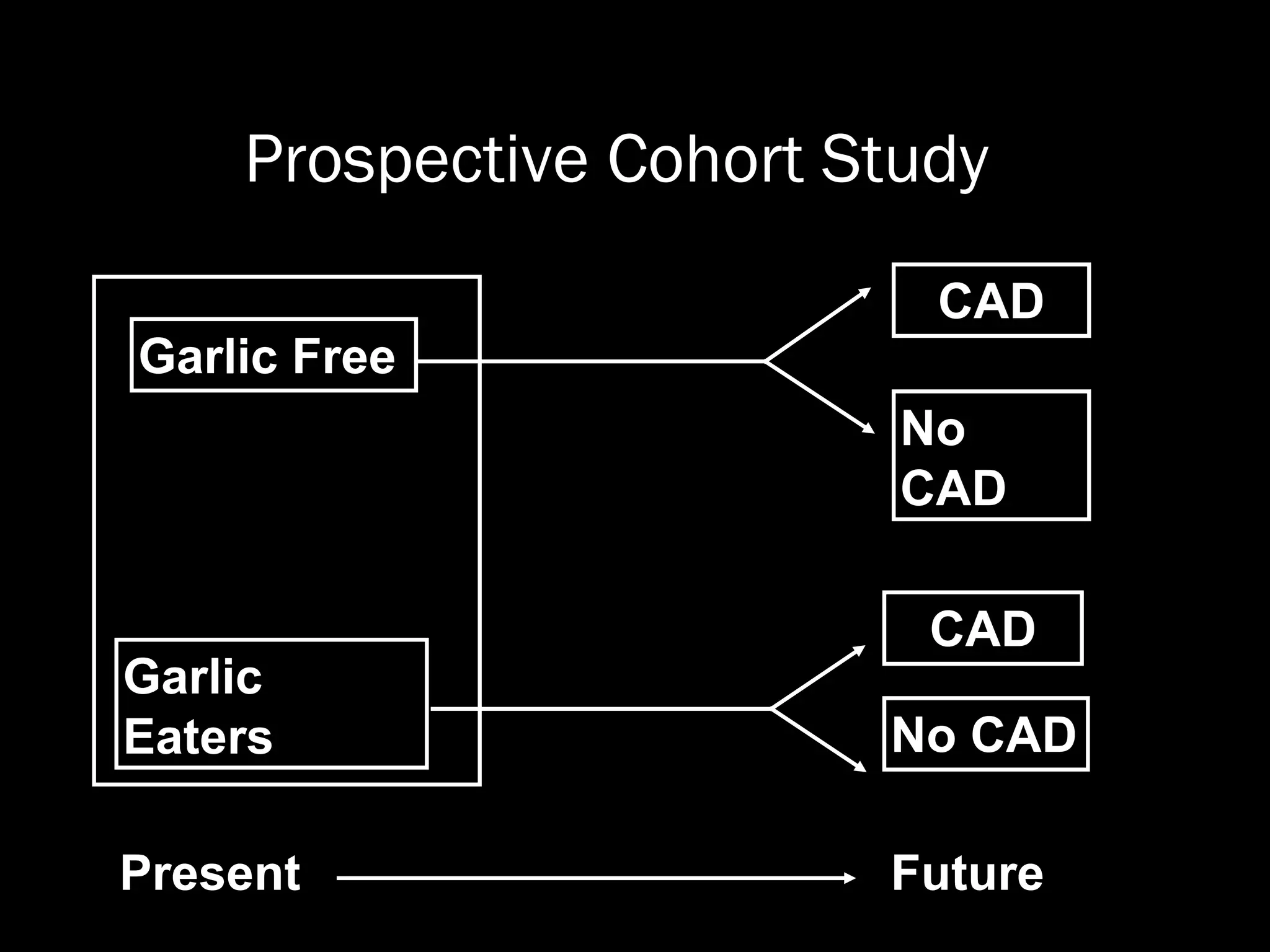
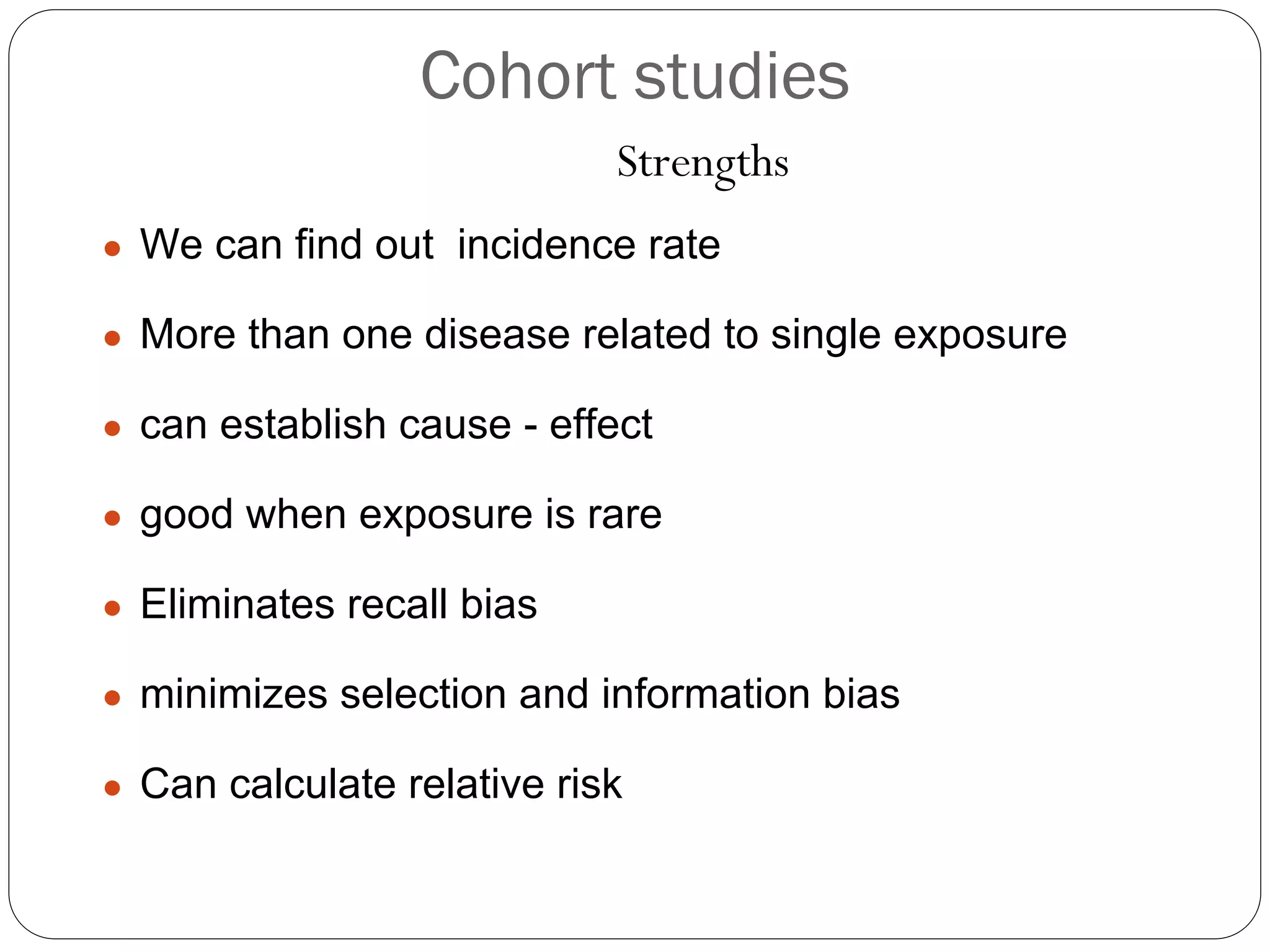
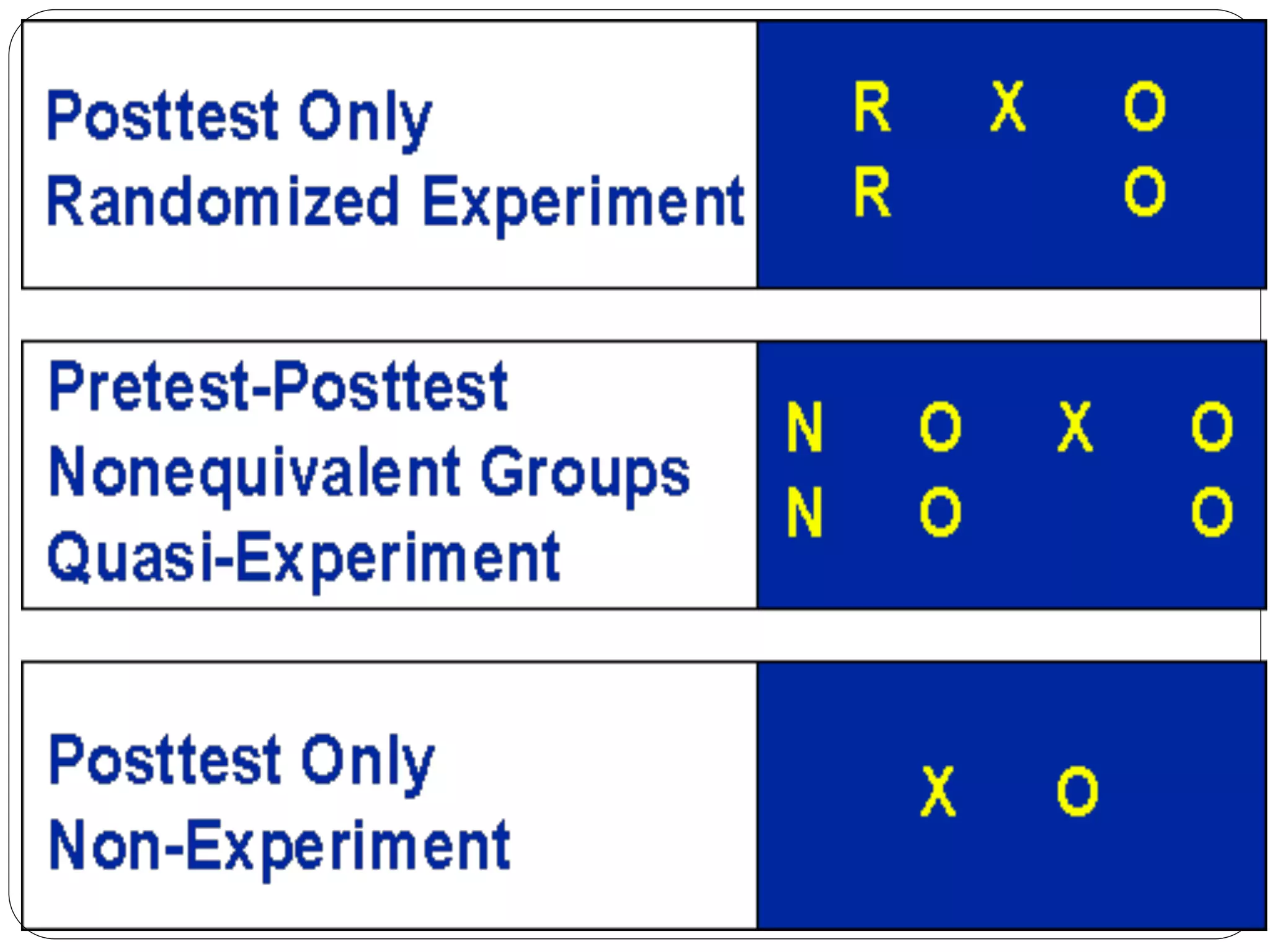
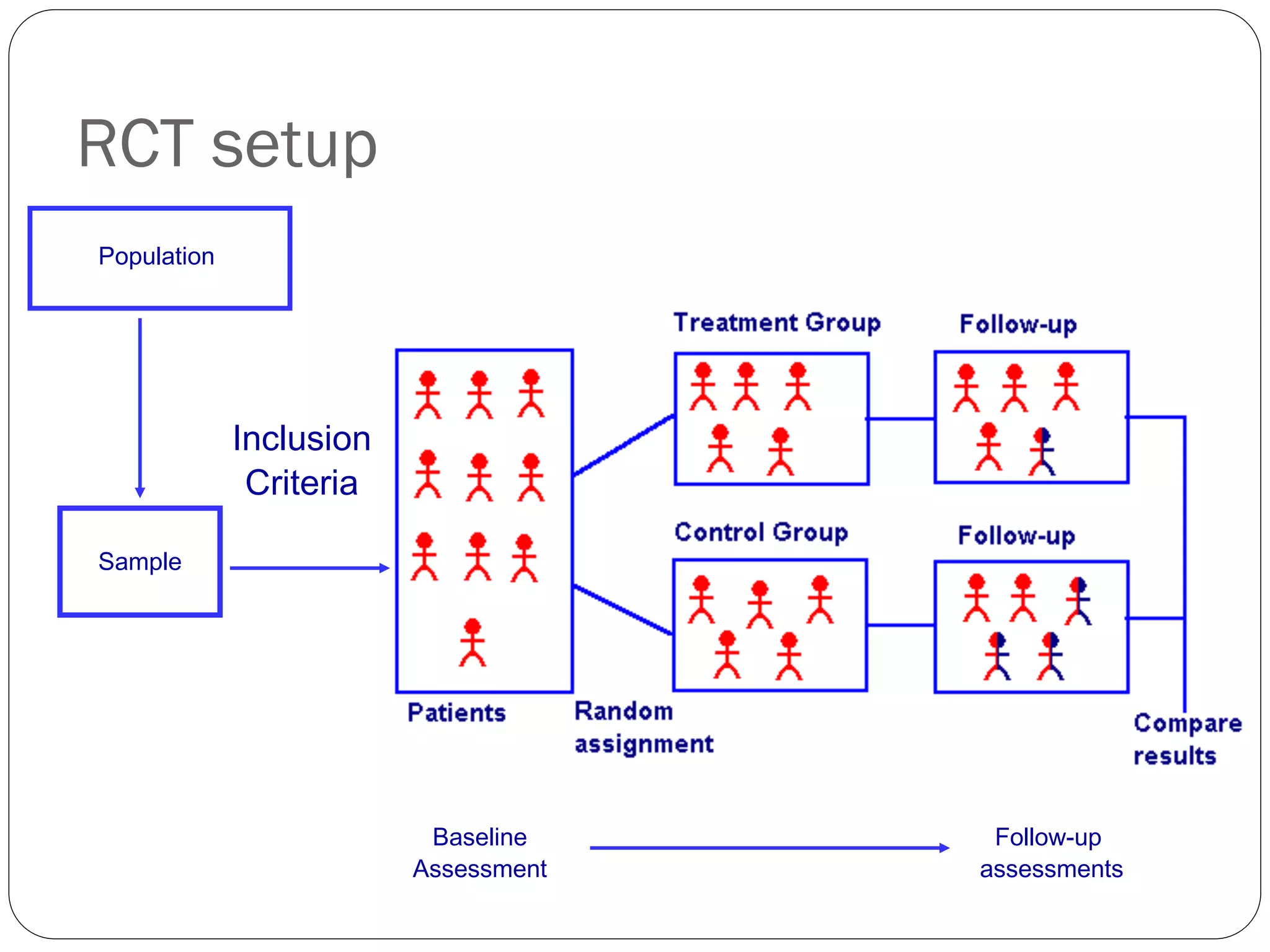
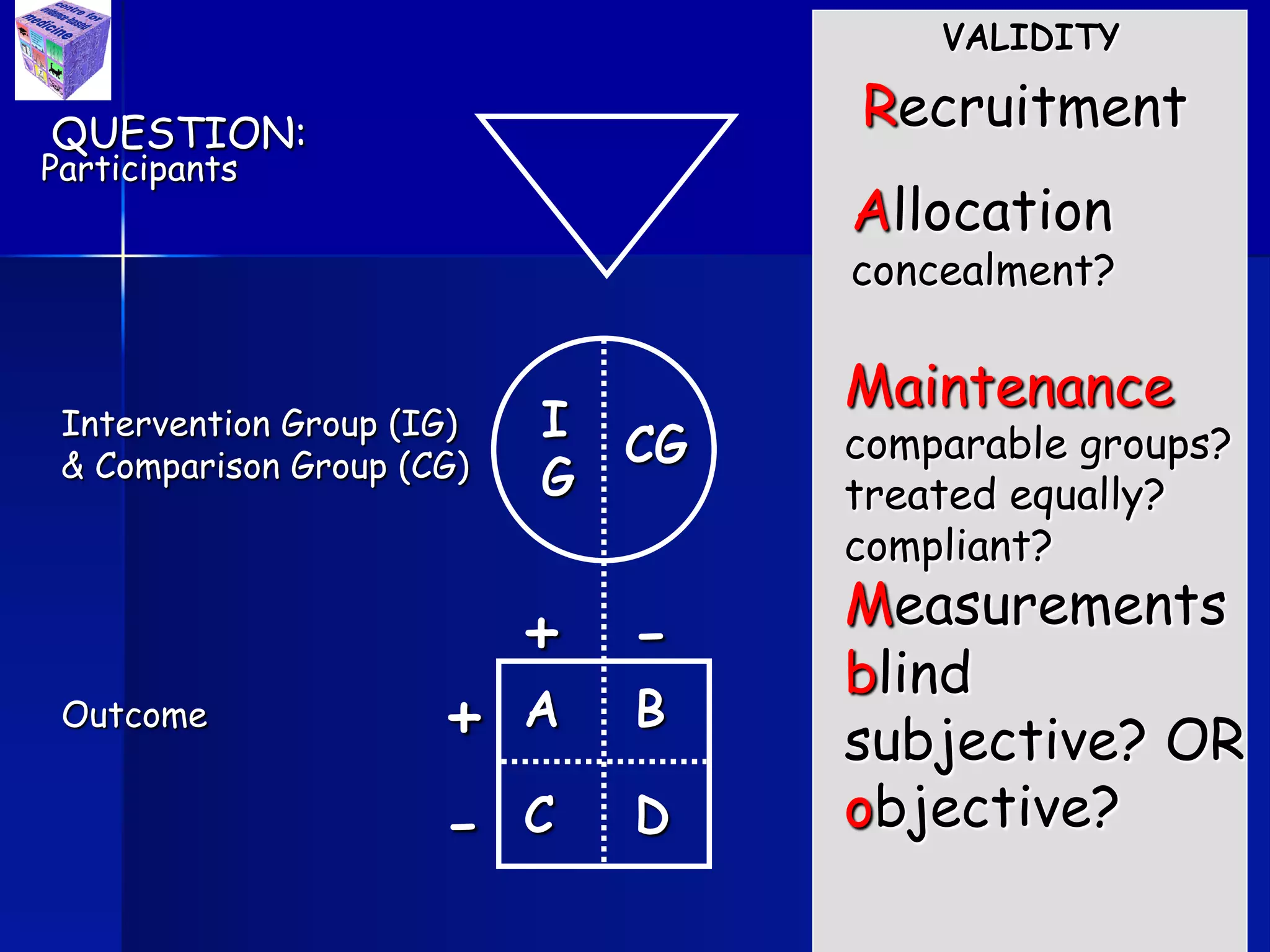
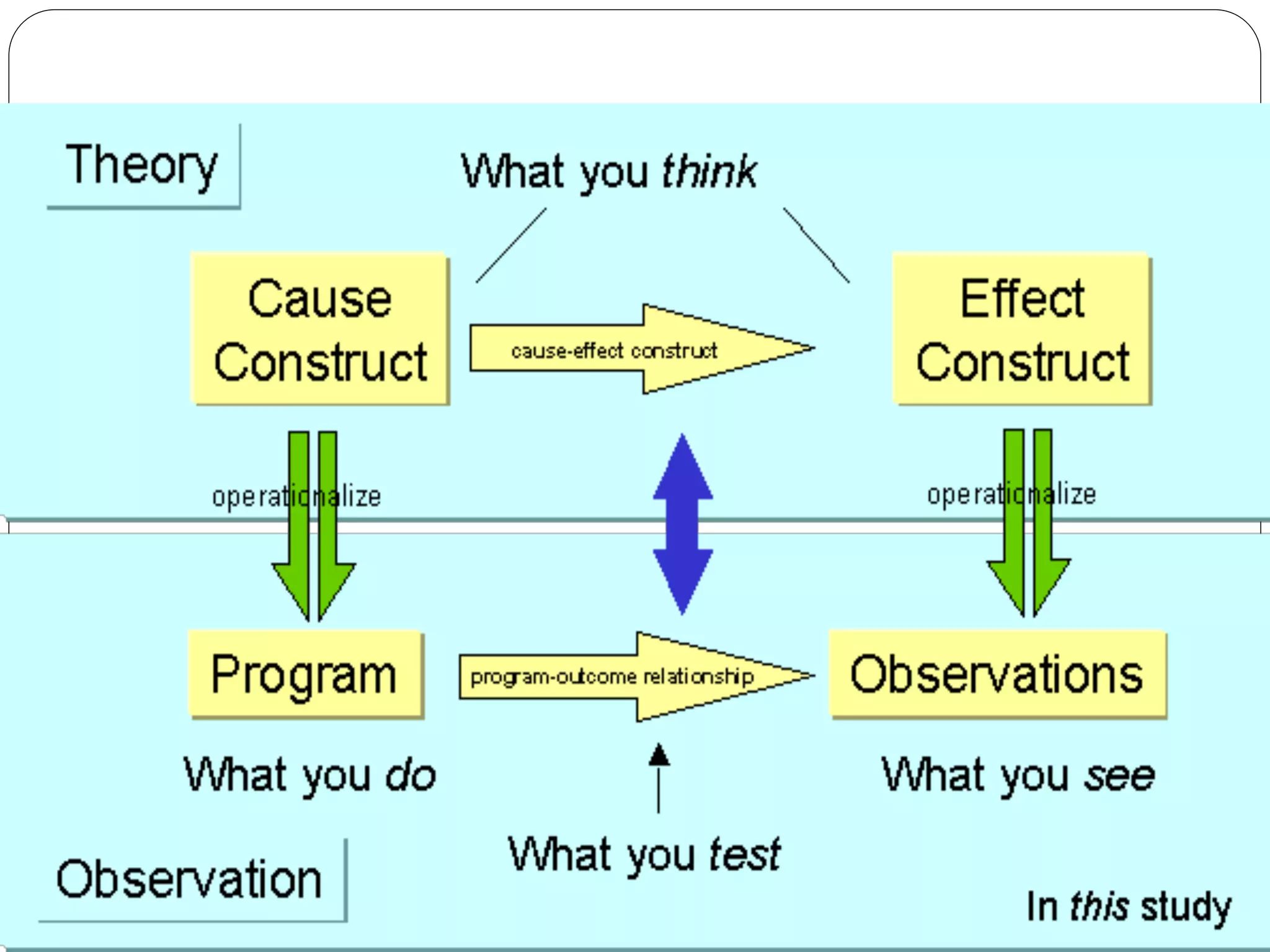
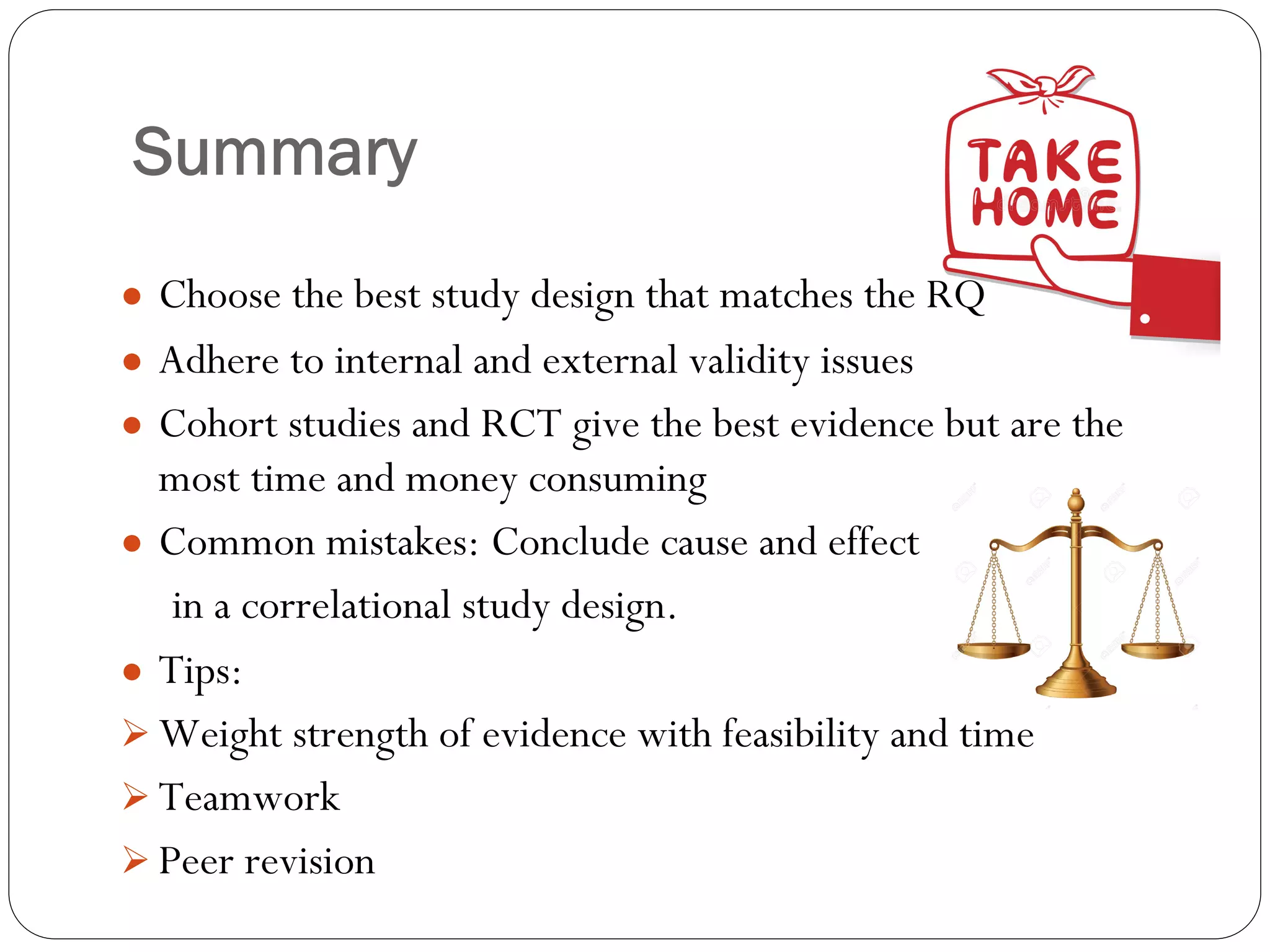
The document outlines key components and considerations for formulating effective research questions (RQs), emphasizing clarity, feasibility, and the capacity for analysis. It discusses types of research questions including descriptive, comparative, and causal, and provides guidelines for developing RQs through various activities. Additionally, it covers research design principles, methodologies, and the importance of clearly defined objectives in conducting research.