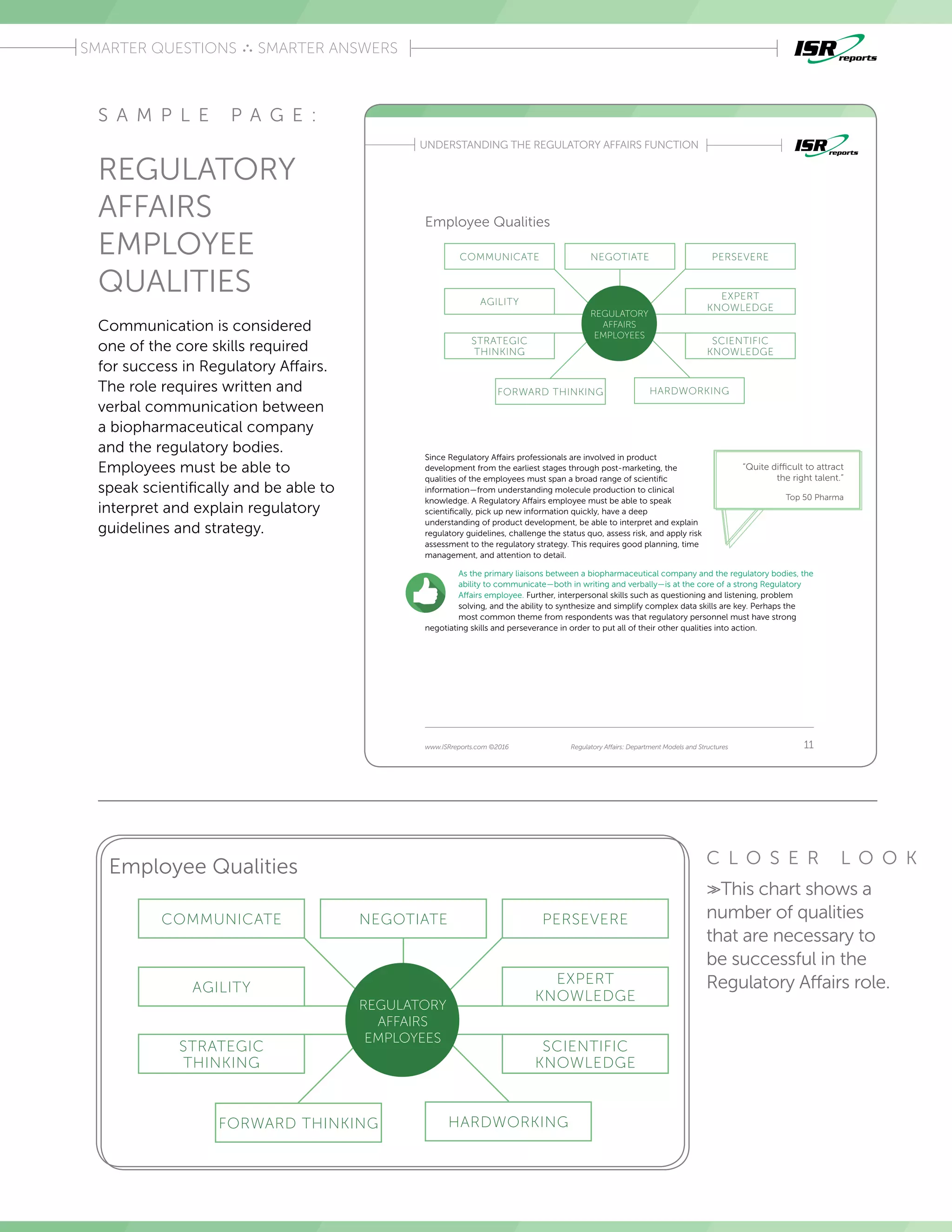
The report examines regulatory affairs functions within the top 50 pharmaceutical companies, based on interviews with 13 industry experts. It aims to serve as a benchmarking tool, highlighting differences in departmental structures, employee qualities, and best practices, along with challenges faced by regulatory affairs teams. Key insights include the importance of communication skills, strategic thinking, and the varying departmental structures that impact budget and operations.