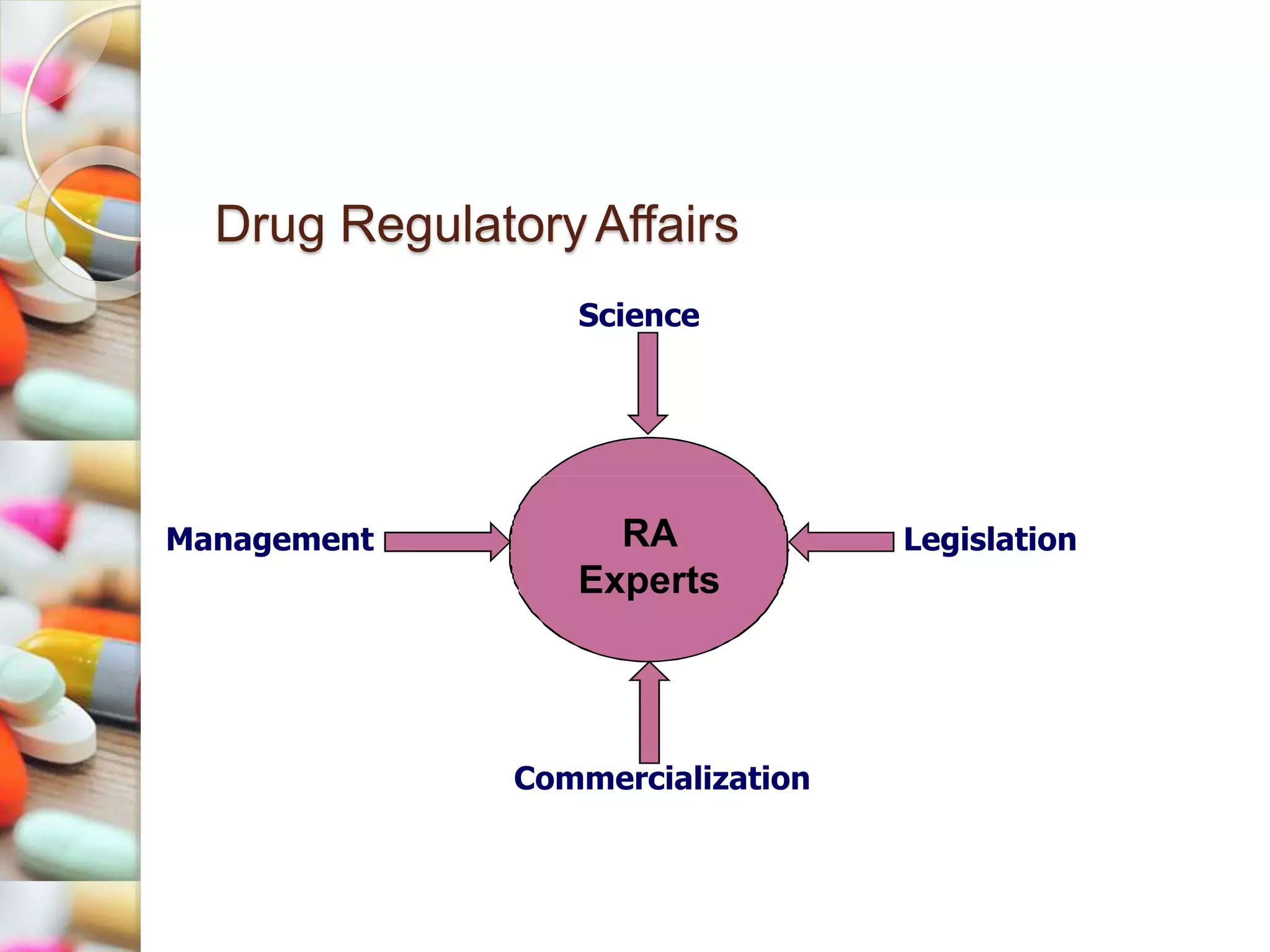
The document discusses Drug Regulatory Affairs (RA), highlighting its importance in ensuring compliance with regulations for pharmaceutical products. It covers the roles and responsibilities of RA experts, the types of companies employing them, and relevant regulatory bodies across different regions. The presentation emphasizes the structured and constantly evolving nature of drug development and the critical role of RA in bringing safe and effective pharmaceuticals to market.