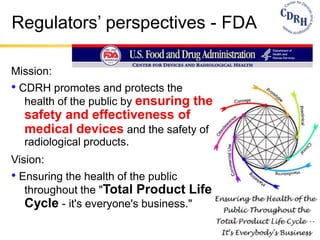
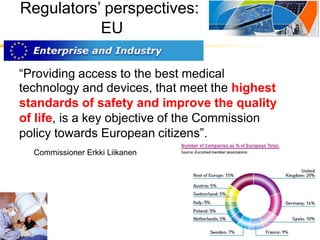
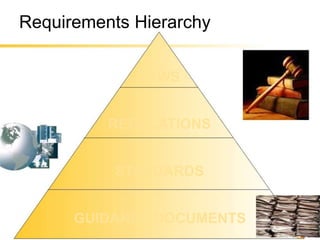
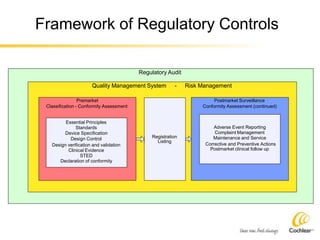
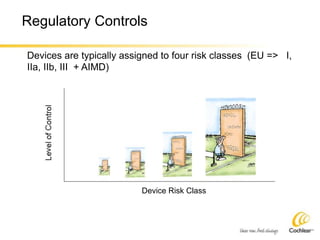
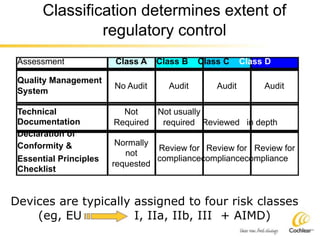
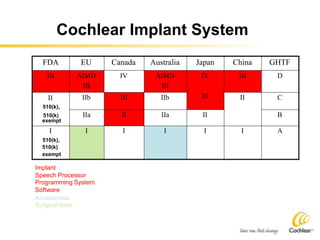
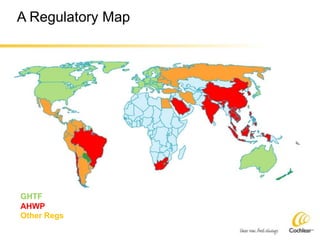
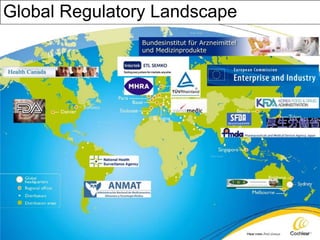
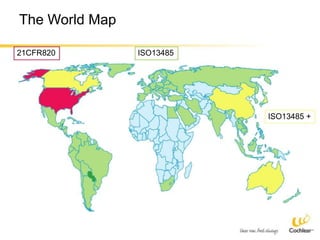
The document provides an overview of medical device regulations from various regulatory agencies around the world. It discusses the purpose of regulations to control human behavior and ensure safety outcomes. Key regulatory agencies discussed include the TGA, FDA, PMDA, and EU, outlining their missions to protect public health through regulatory excellence and appropriate controls. A regulatory framework hierarchy is presented from laws to standards to guidance. Classification determines the extent of control and conformity assessments required. Cochlear implant systems are classified differently by various agencies. The key regulations governing Cochlear are also listed.







![Key Regulations for Cochlear
Regulations
1. Therapeutic Goods (Medical Devices) Regulations 2002 (Statutory Rules 2002 No. 236), Therapeutic Goods
Administration, Australia
2. Uniform Recall Procedure for Therapeutic Goods 2004 edition (Australia)
3. Food and Drug Administration, USA Title 21, Code of Federal Regulations
Part 820: Quality System Regulation.
Part 11 Electronic Records, Electronic Signatures
Part 806 Medical Devices, Reports of Corrections and Removals
Part 803 Medical Device Reporting
Part 812 Investigational Device Exemptions
Part 814 Premarket Approval of Medical Devices
4. MHLW (Ministry of Health, Labour, and Welfare, Japan) Ordinance no. 169 (revised GMP, Japan) based on
PAL (Pharmaceutical Affairs Law, Japan) [Law No. 145, as of August 10, 1960; Law No. 87 as of July 26,
2005]
5. Active Implantable Medical Device Directive (AIMD) 90/385/EEC (including 2007 rev), Europe
6. Medical Device Directive (MDD) 93/42/EEC (including 2007 rev), Europe
7. Medical Device Regulations (SOR/98-282) 1998, Department of Justice, Canada
8. Regulations for the Supervision and Administration of Medical Devices, State Food and Drug Administration
(SFDA), China
9. Medical Devices Regulation, KFDA. Korea Food and Drug Administration, Korea
10. Medical Devices Regulation, Taiwan Department of Health DoH, Taiwan](https://image.slidesharecdn.com/regsummary-130625005912-phpapp01/85/Reg-summary-20-320.jpg)