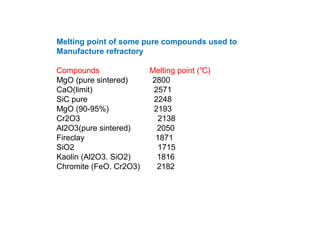
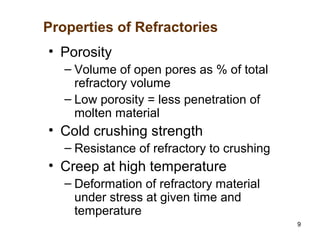
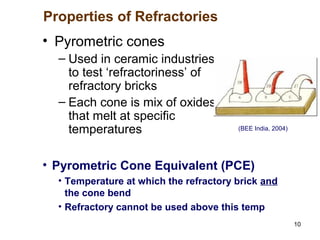
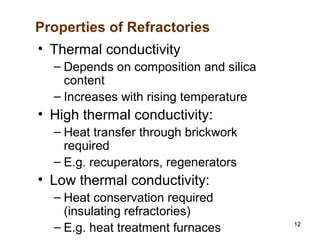
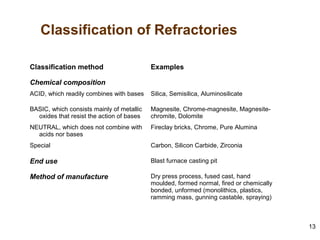
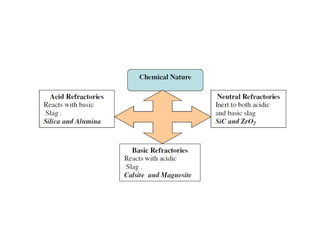
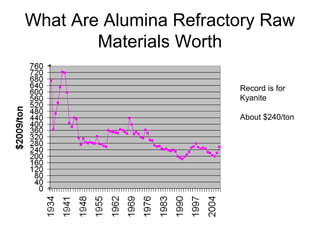
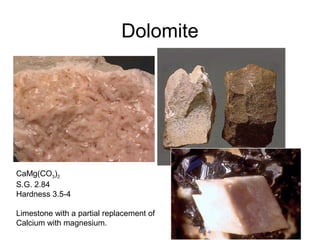
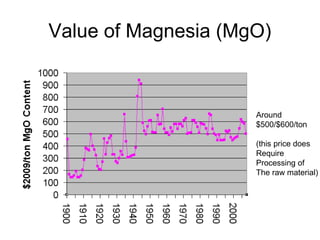
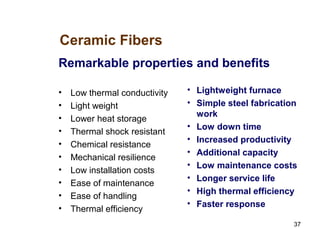
Refractory materials are required for steelmaking as they can withstand very high temperatures without undergoing chemical changes. They are needed to contain molten steel, slag and hot gases. High quality refractories are important to reduce costs as the refractory material costs are added to the overall product costs. Refractories must withstand high temperatures, thermal shocks, chemical reactions with molten materials, and abrasion. Common refractory materials include fireclay, magnesia, chromite, and alumina, which are selected based on the temperature and chemical environment they will be used in. Newer refractory application methods like monolithics and high emissivity coatings provide benefits over traditional refractory bricks.