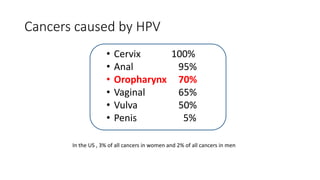
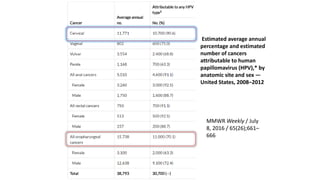
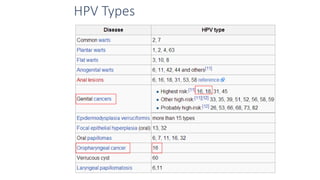
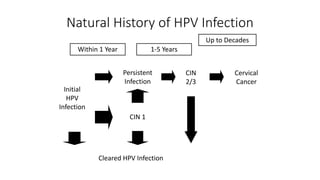
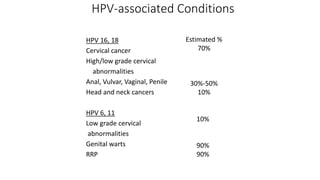
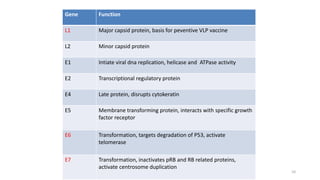
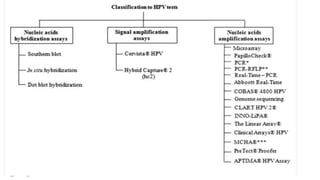
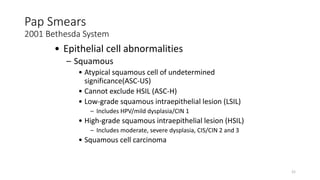
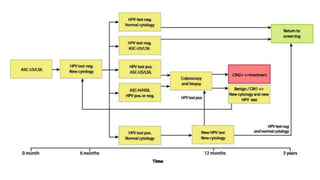
- Human papillomavirus (HPV) is a non-enveloped double-stranded DNA virus that can cause various cancers and genital warts. There are over 100 HPV types, with high-risk HPV 16 and 18 causing most cervical and other cancers.
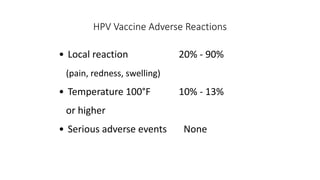
- The HPV vaccines Gardasil (HPV4) and Cervarix (HPV2) protect against HPV 16 and 18 and can prevent cancers caused by these high-risk types if administered before exposure. Vaccination is recommended for girls and boys at age 11-12.
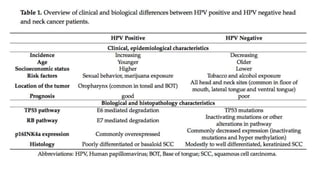
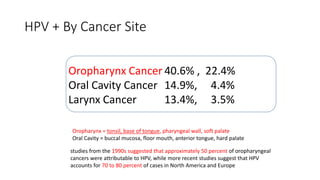
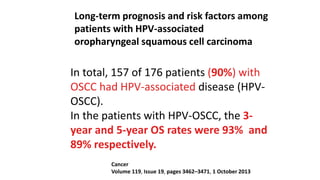
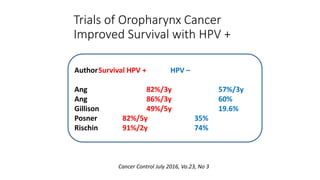
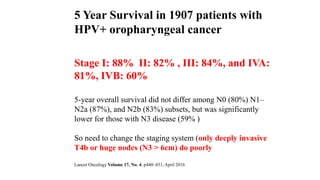
- HPV positive oropharyngeal cancer makes up the majority of oropharyngeal cancers and is associated with improved survival compared to HPV negative orophary