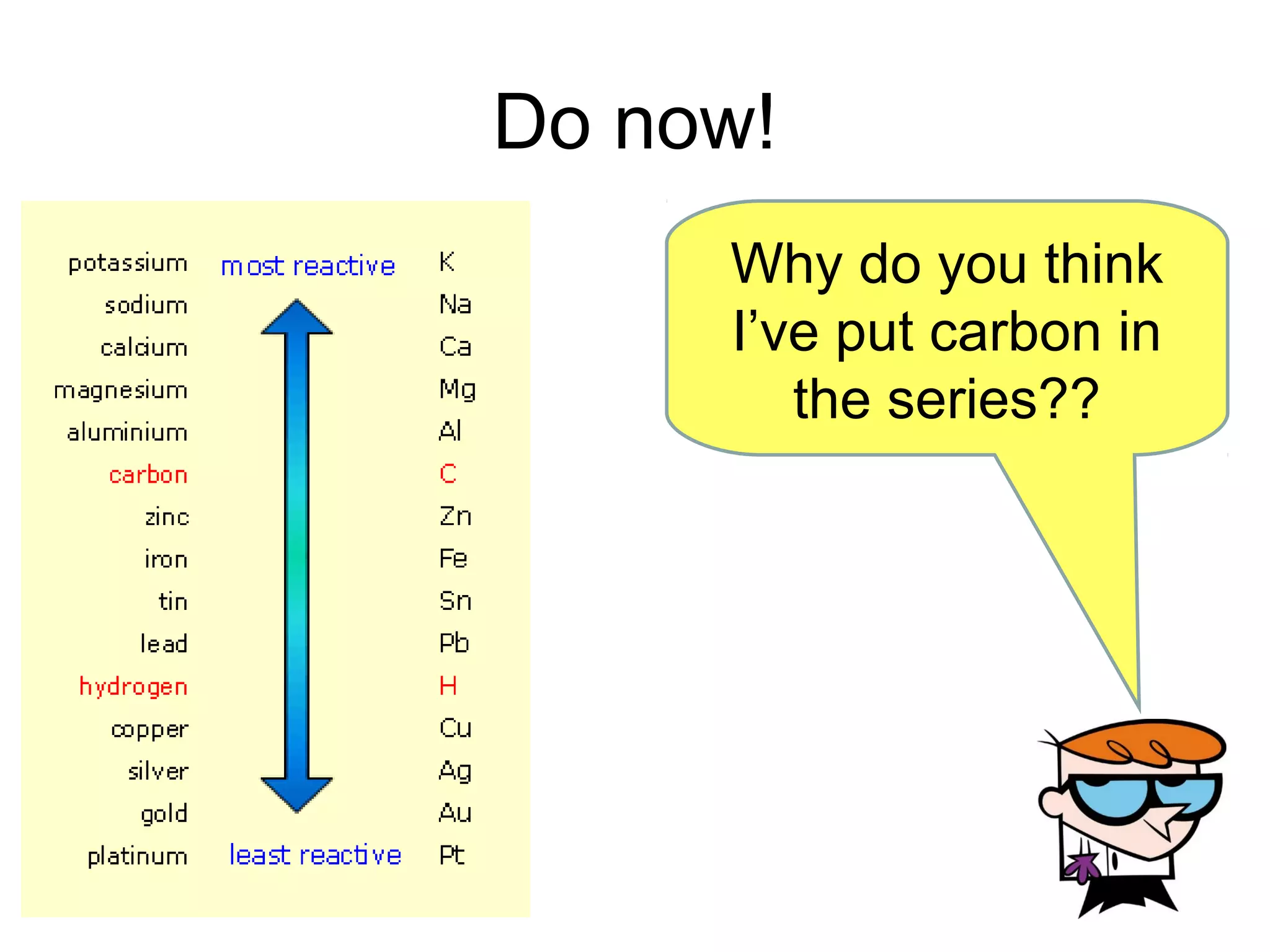
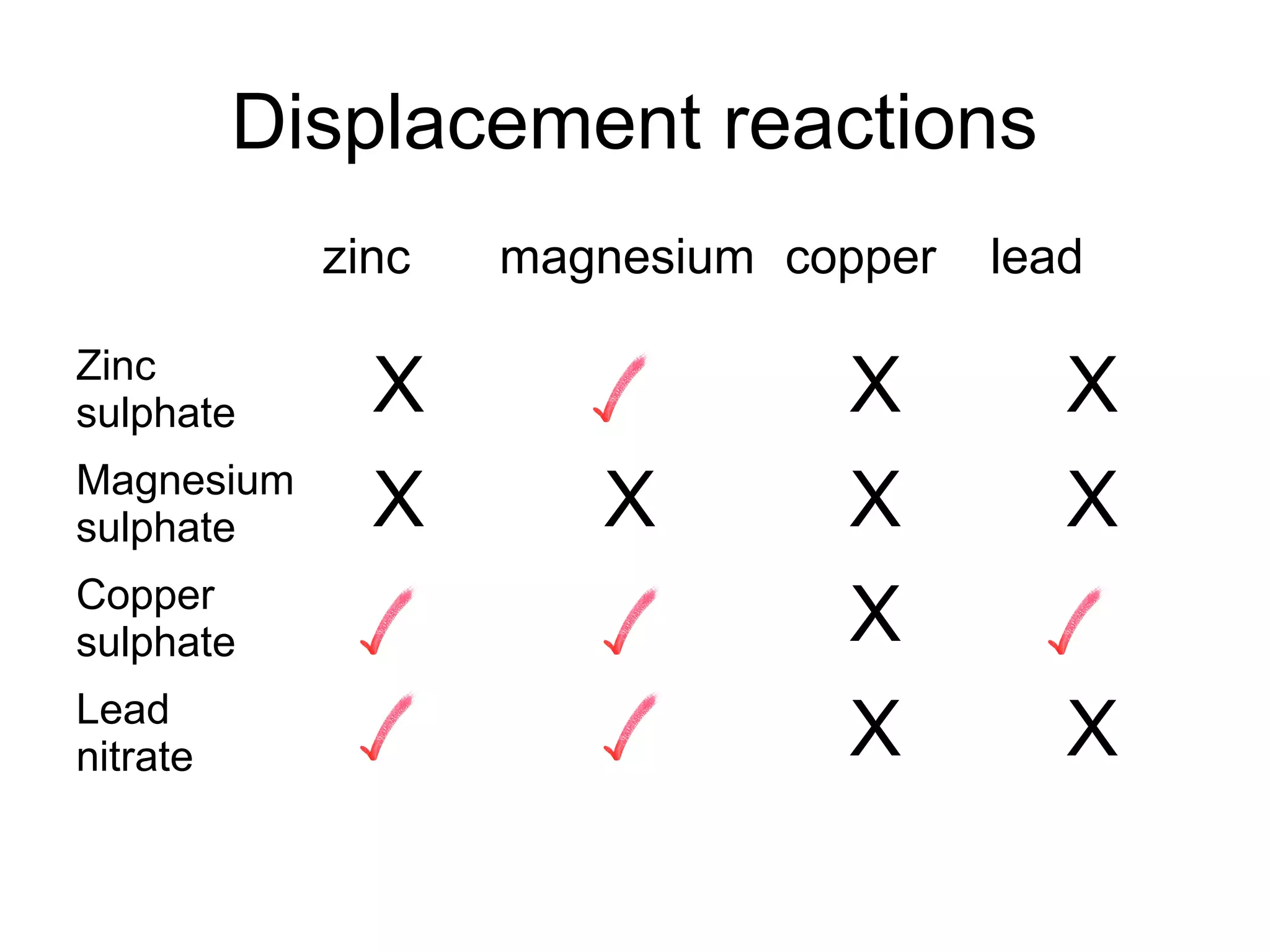
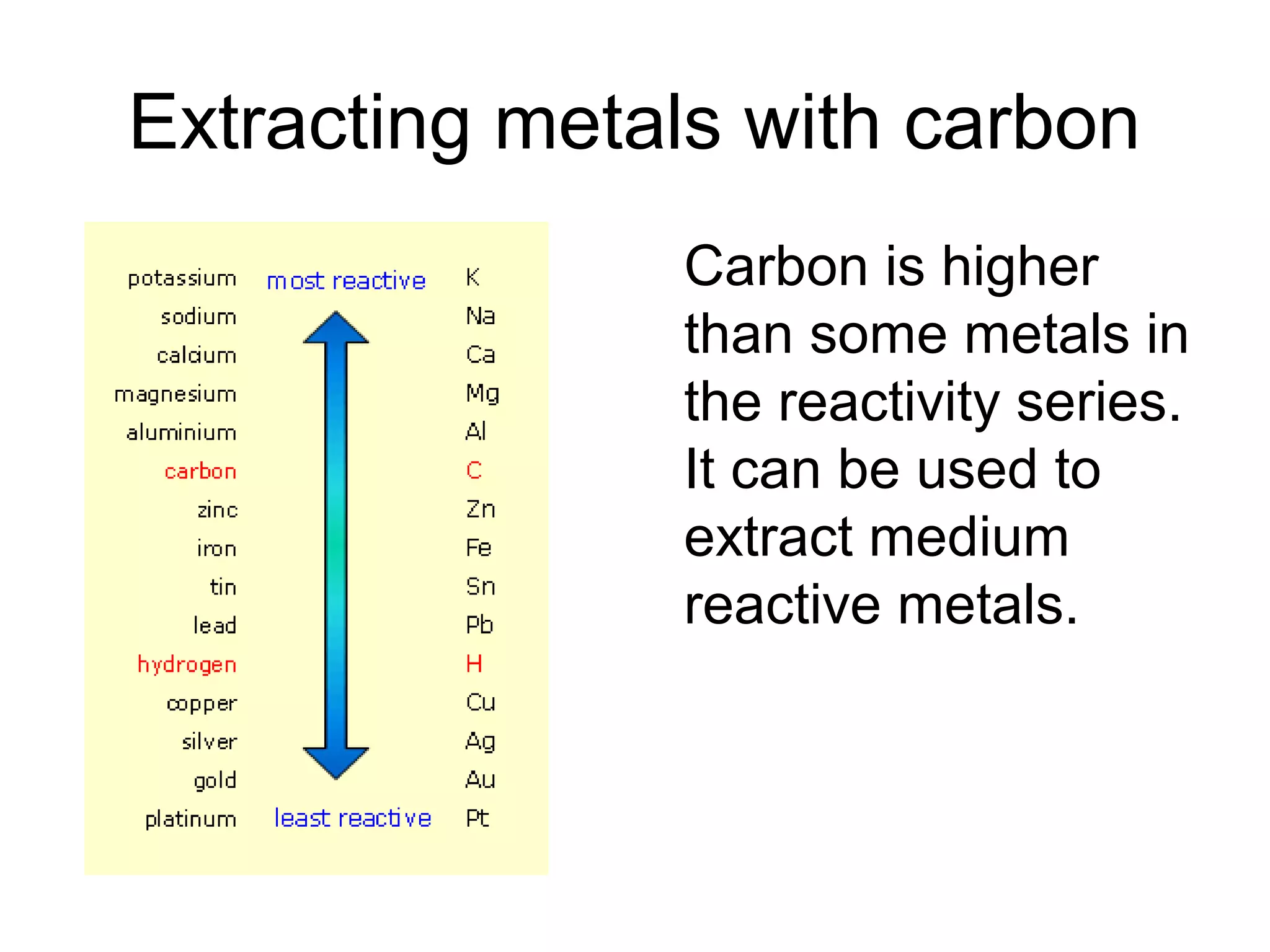
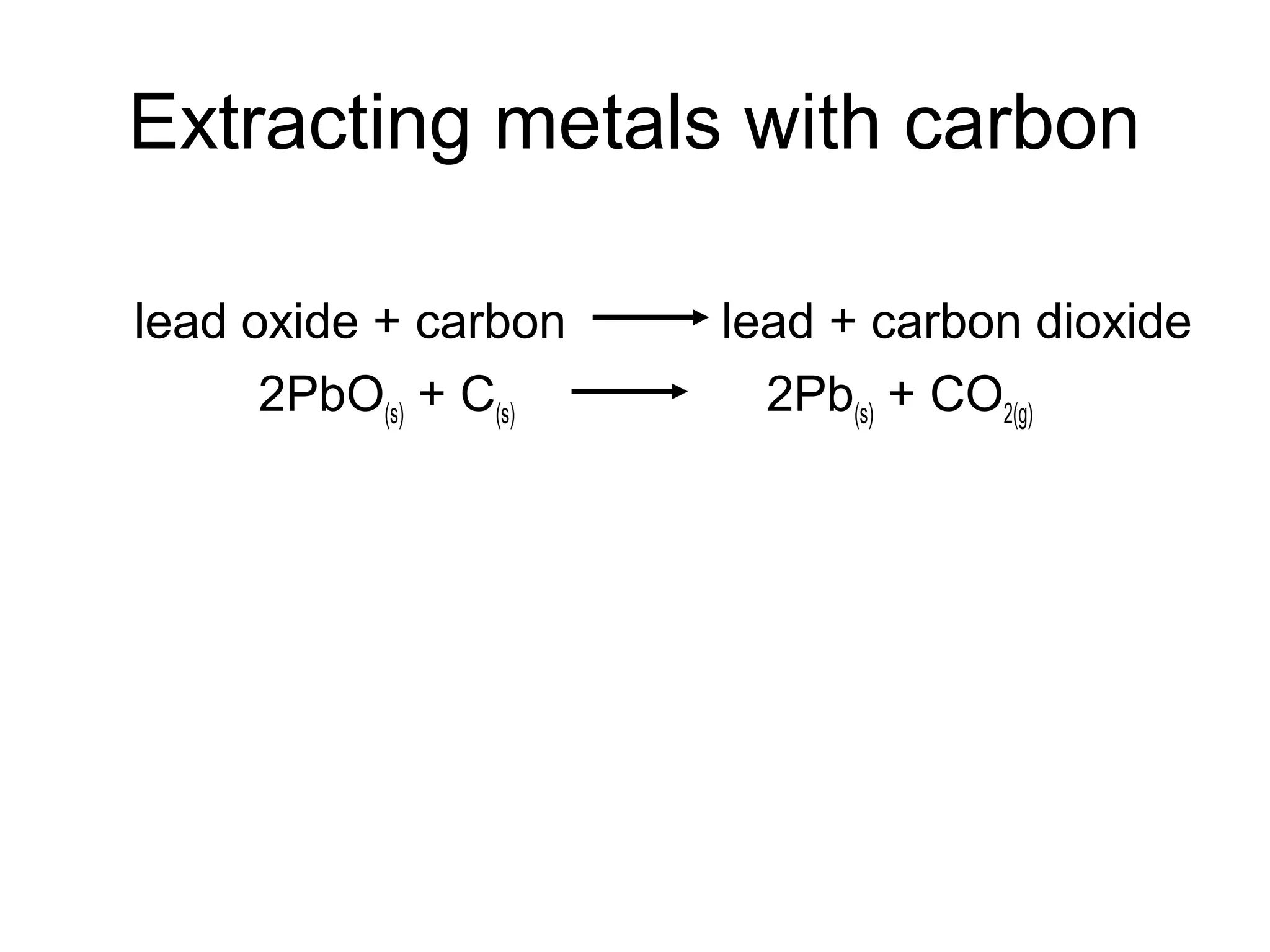
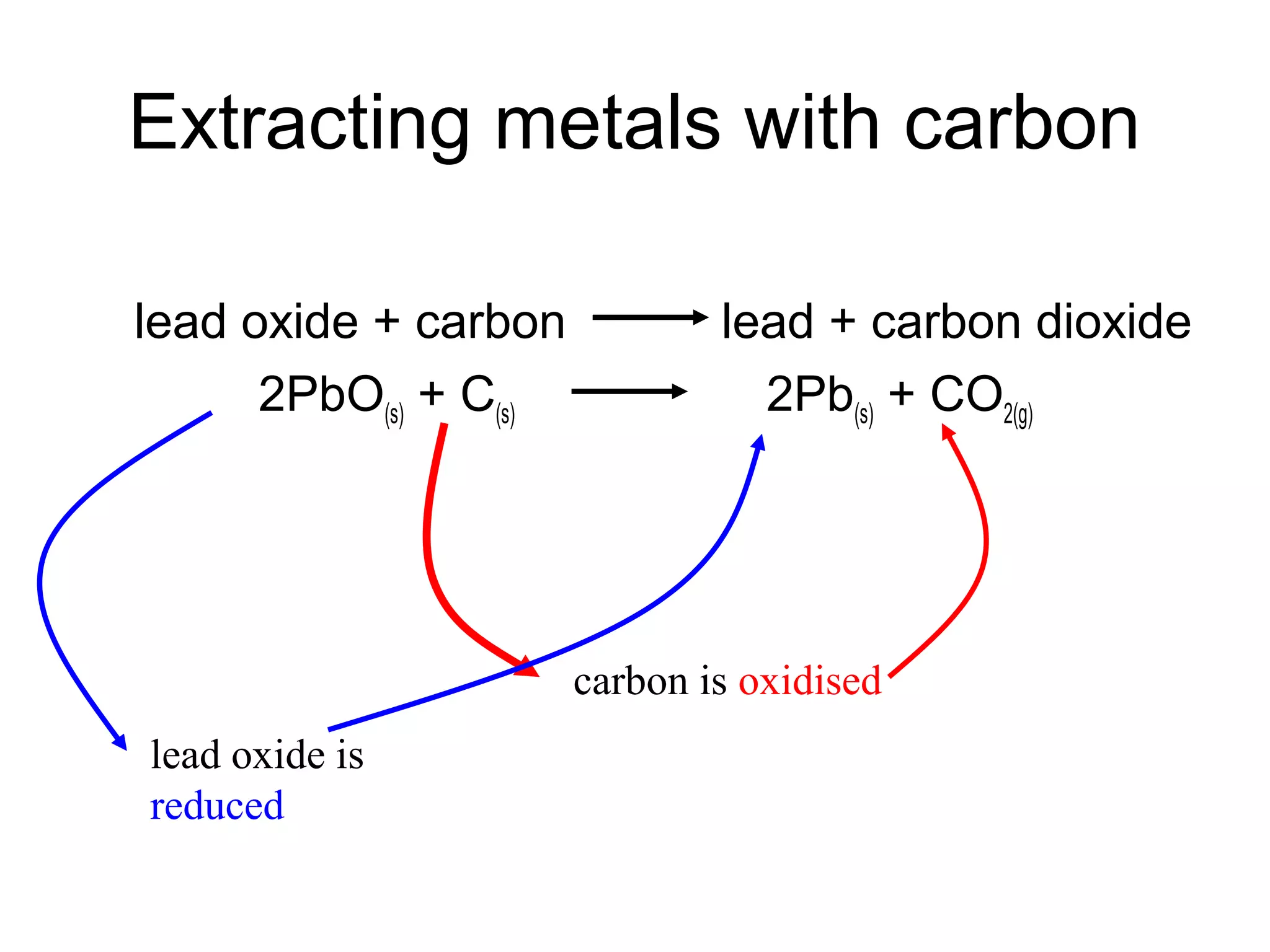
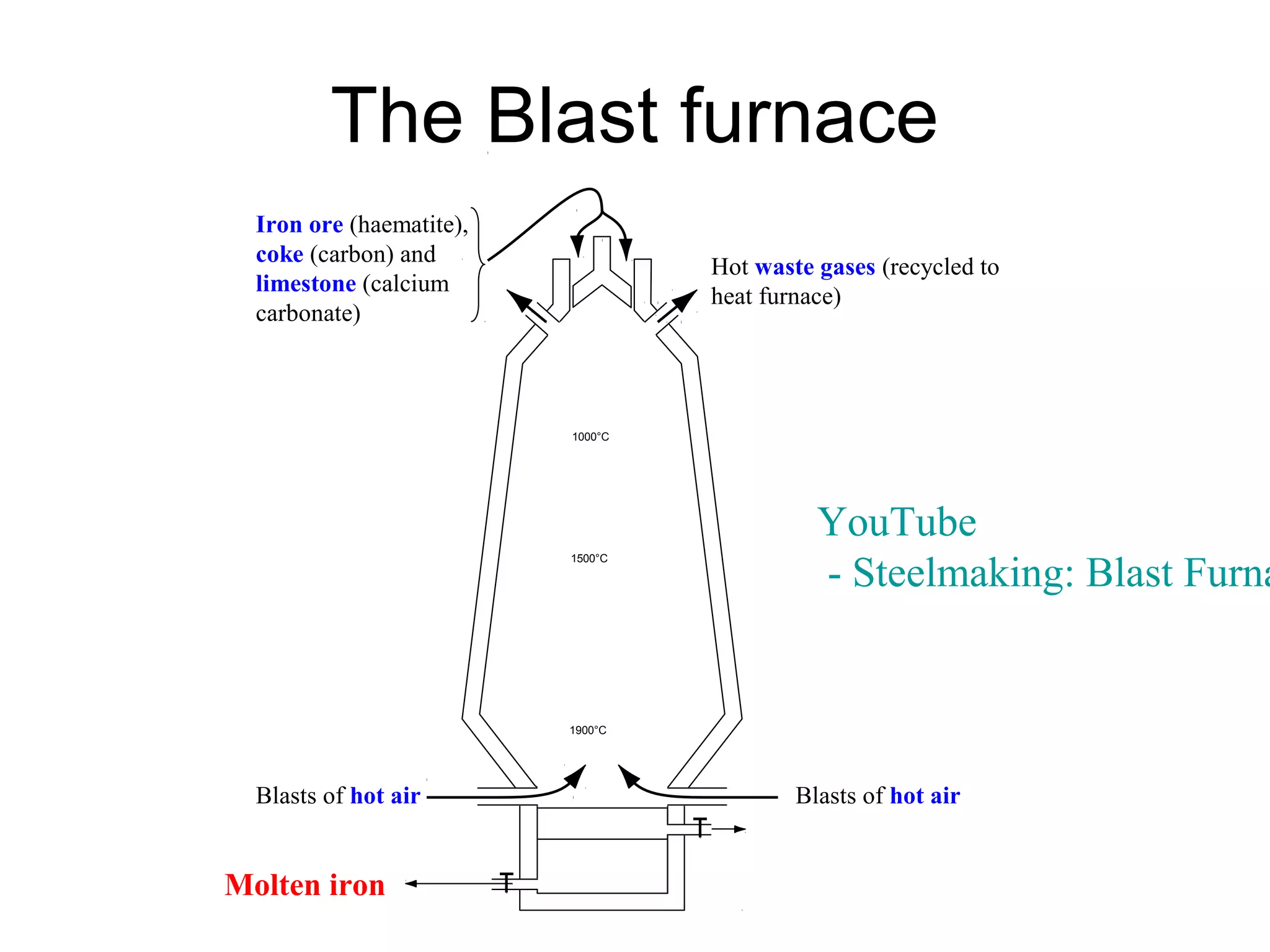
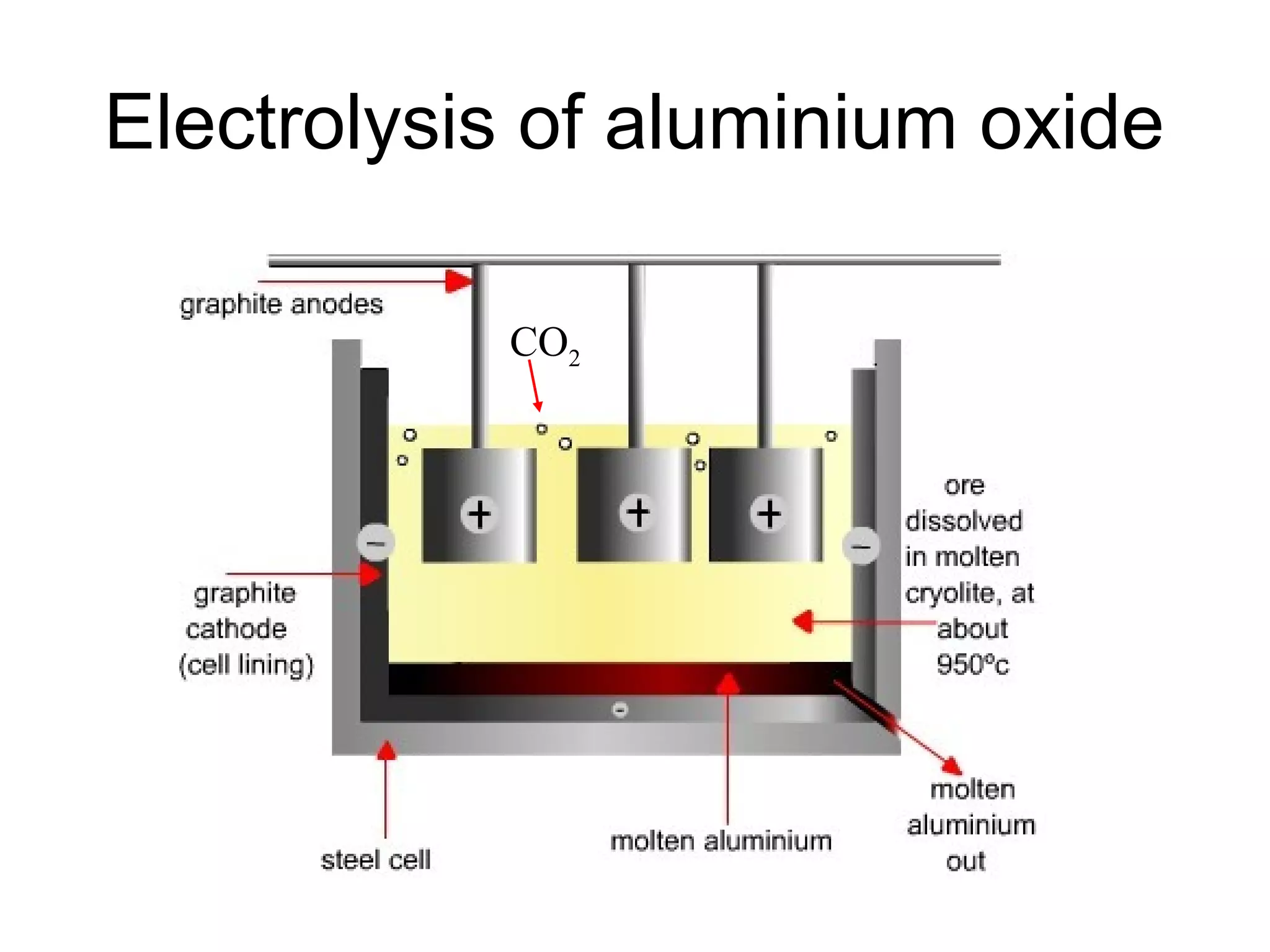
The document discusses extracting metals from ores and their uses. It explains that more reactive metals can displace less reactive metals in solutions and that carbon can be used to extract some metals by reducing metal oxides to metals. Examples are given of extracting lead through heating lead oxide with carbon to produce lead and carbon dioxide. The extraction of iron in a blast furnace is also summarized. Finally, common uses of extracted metals like gold, aluminum, steel, and copper are listed.