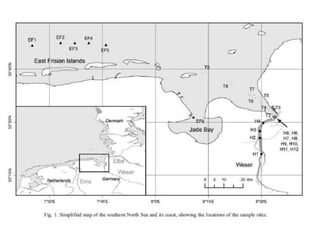
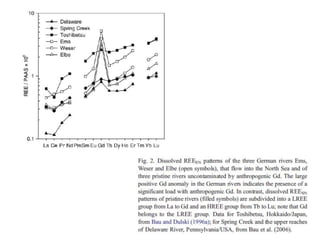
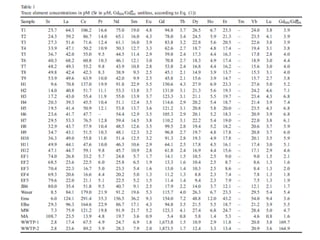
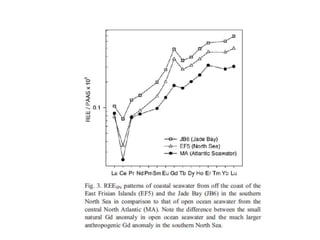
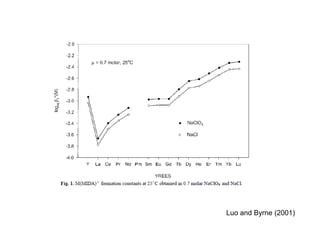
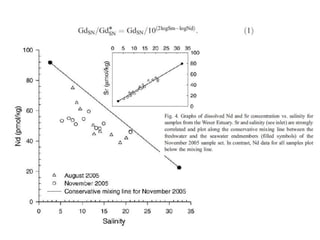
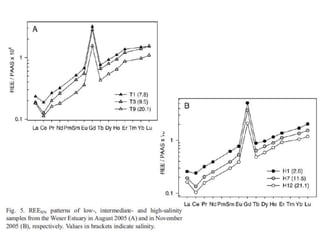
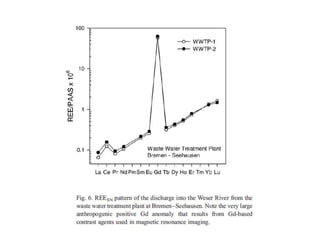
The Rare Earth Elements
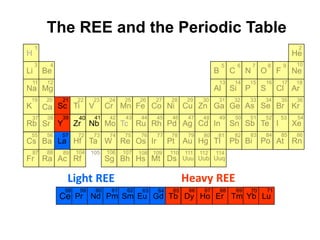
The rare earth elements (REE) are a group of 17 elements that appear together on the periodic table. Three key points about REE:
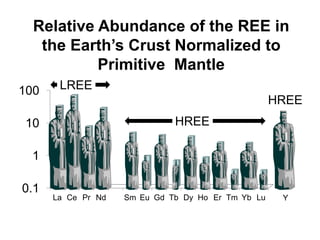
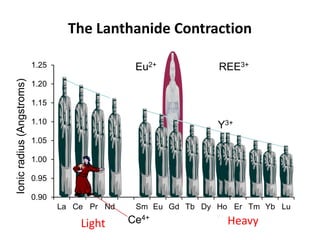
1. Light REE (LREE) are more abundant in the Earth's crust than heavy REE (HREE) due to the lanthanide contraction trend of decreasing ionic radius across the REE series.
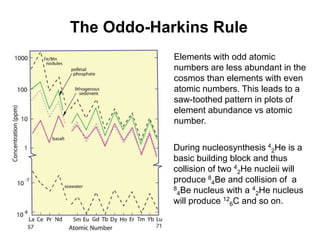
2. Elements with odd atomic numbers are generally less abundant than even atomic numbers due to nucleosynthesis processes (Oddo-Harkins rule).
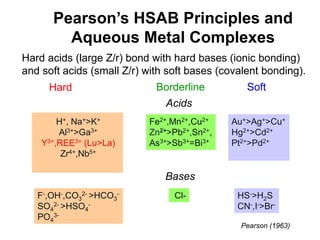
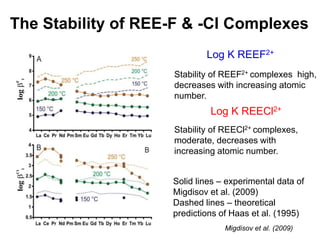
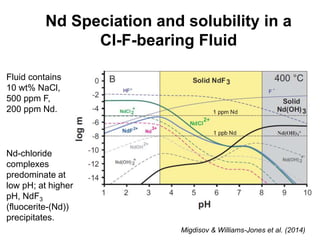
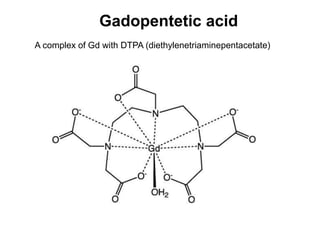
3. REE form stable aqueous complexes with ligands like fluoride and chloride depending on factors like pH, atomic number, and ligand type according to Pearson's HSAB principles