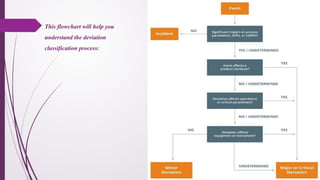
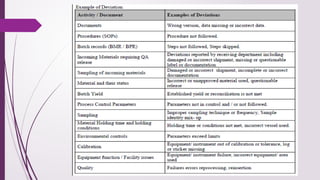
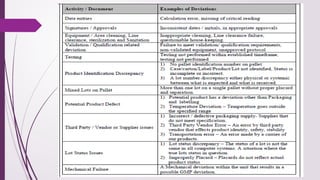
The document defines deviations in life sciences as unwanted events differing from approved processes, categorized as planned or unplanned deviations, and emphasizes the systematic approach of deviation management, including identification, reporting, investigation, documentation, and implementation of corrective actions. It outlines the classification of deviations into critical, major, and minor categories based on their impact on product quality and patient safety, along with the importance of having clear policies, roles, and training in place for effective management. Additionally, the text distinguishes between deviations and incidents, clarifying that deviations involve non-compliance with established protocols while incidents are uncontrolled events unlikely to affect product quality directly.