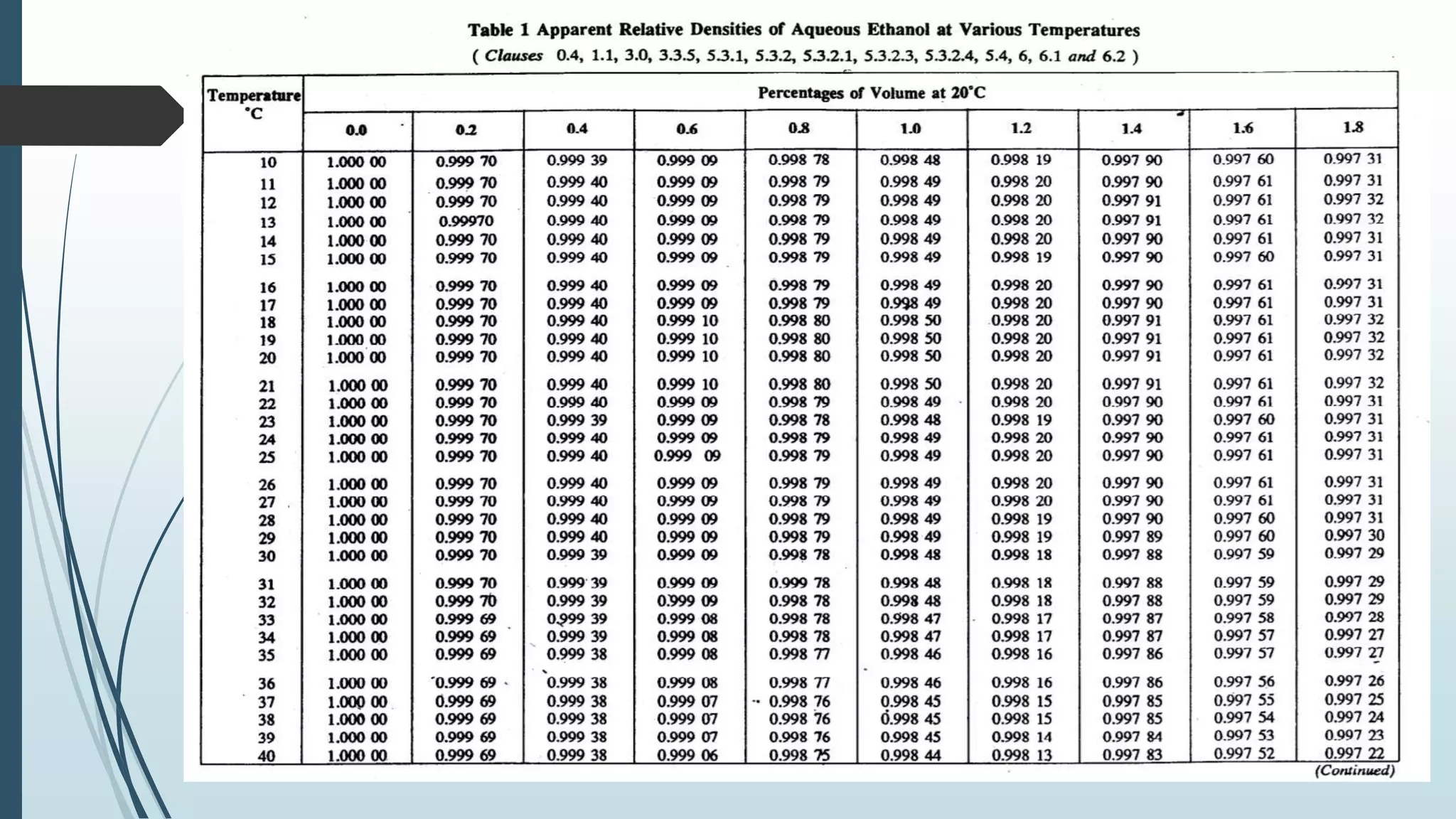
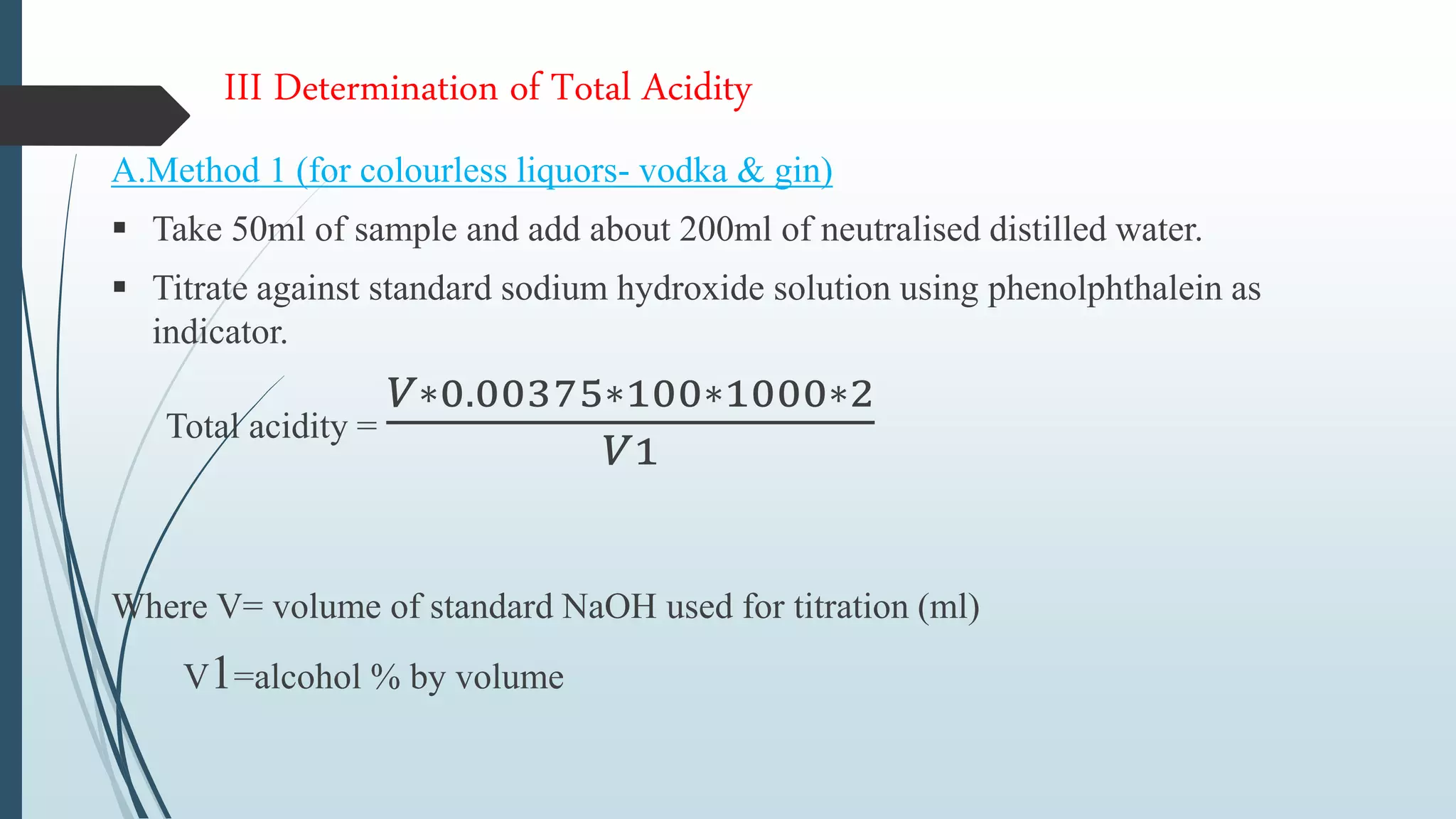
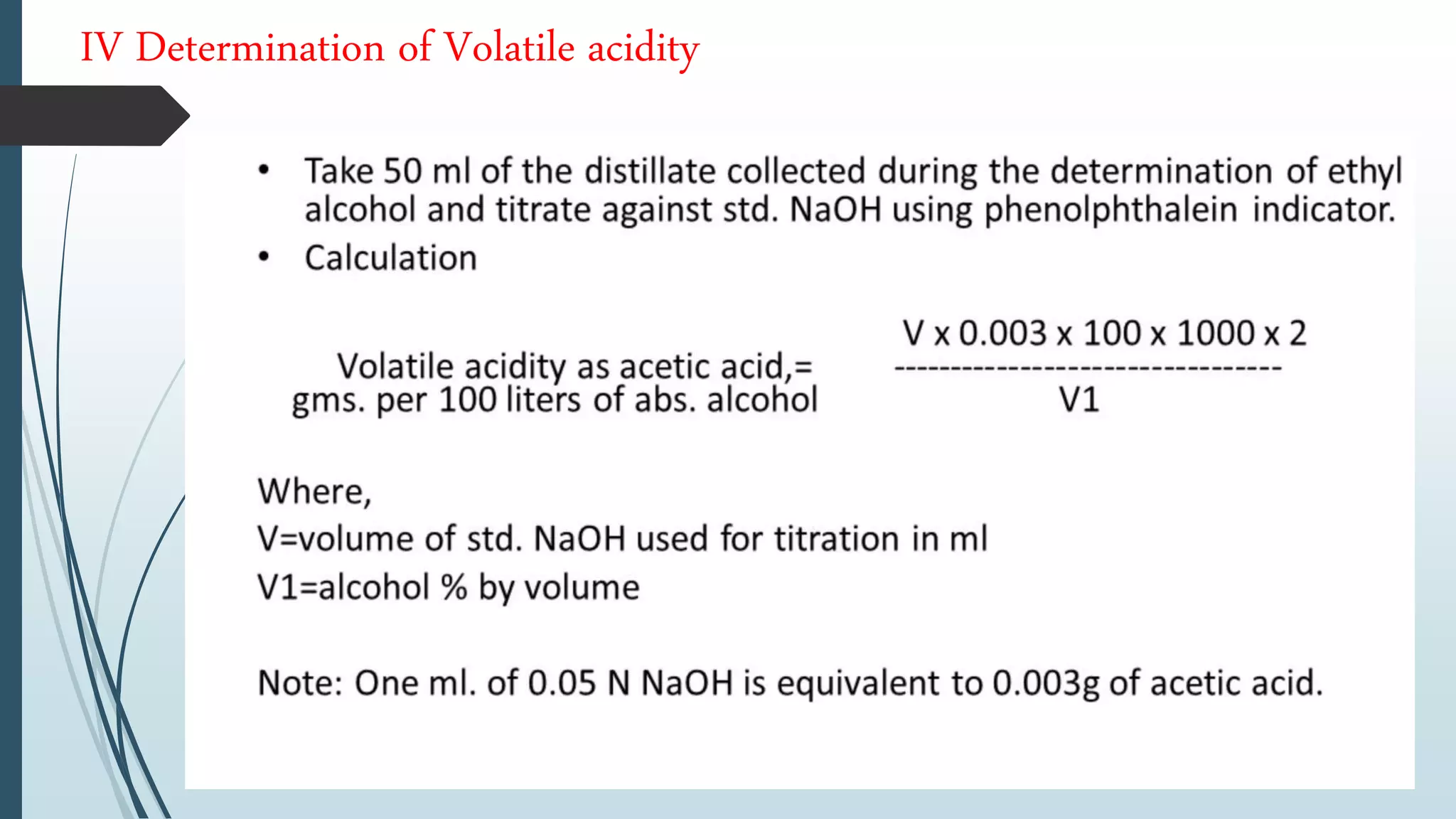
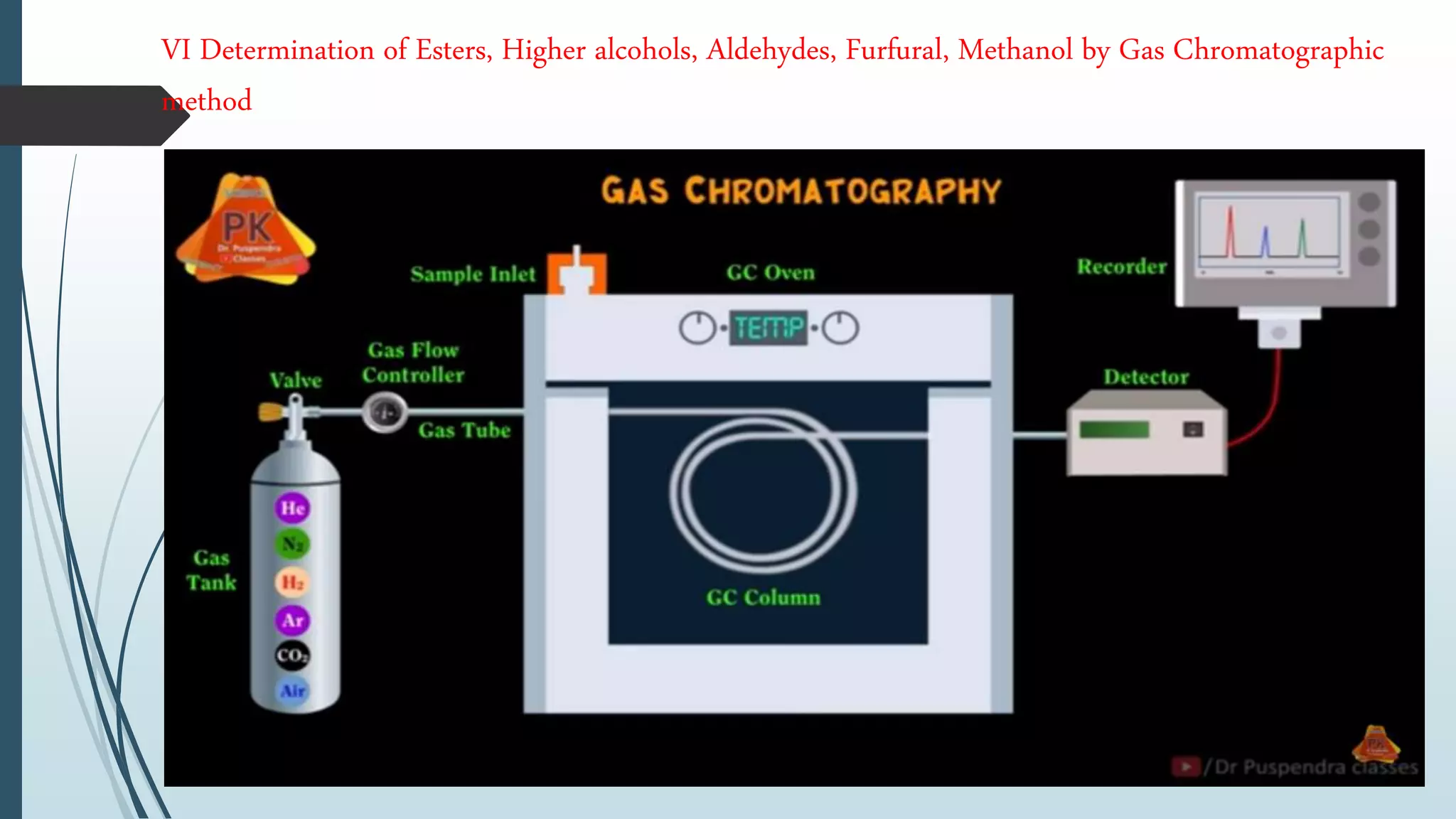
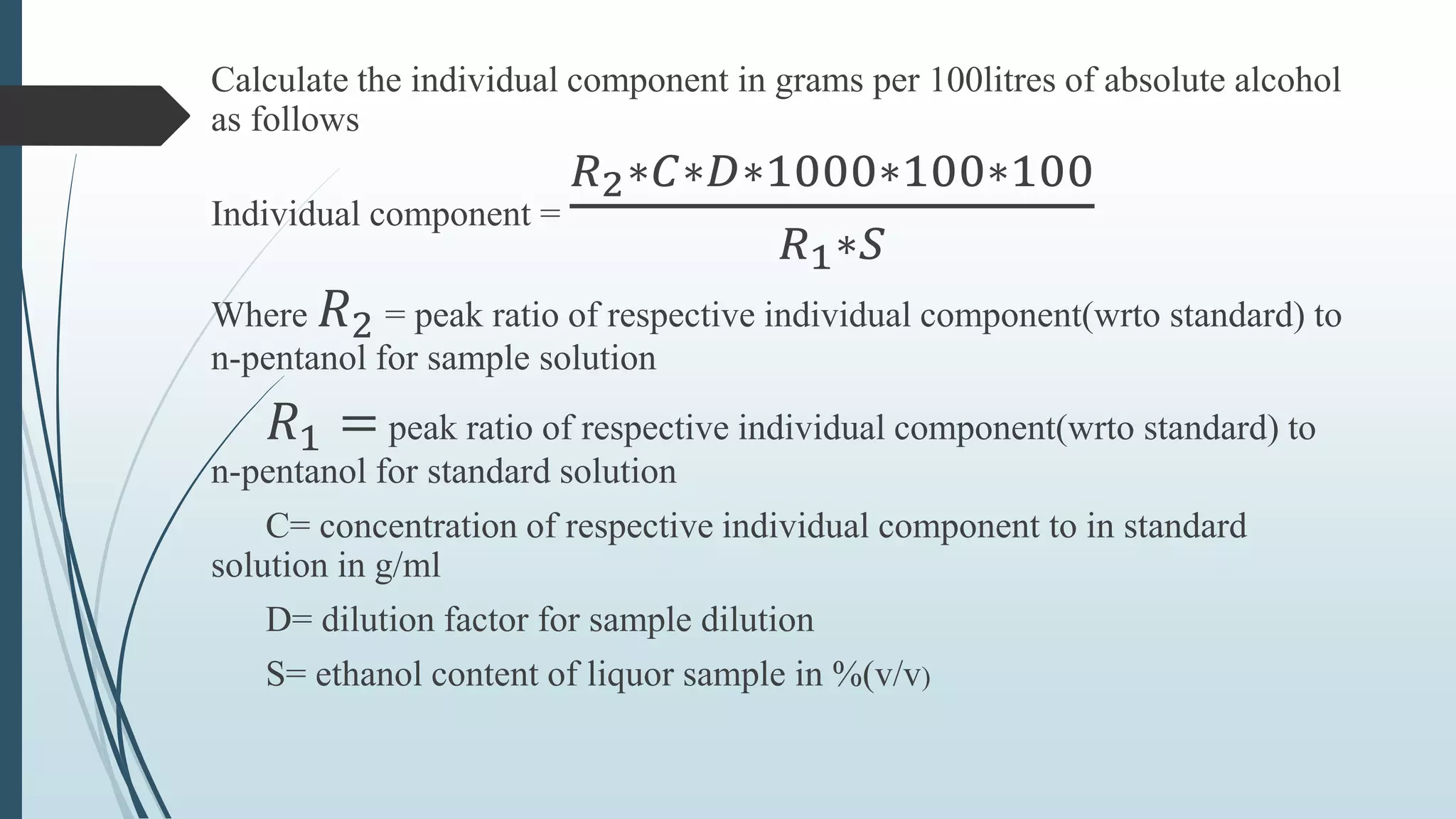
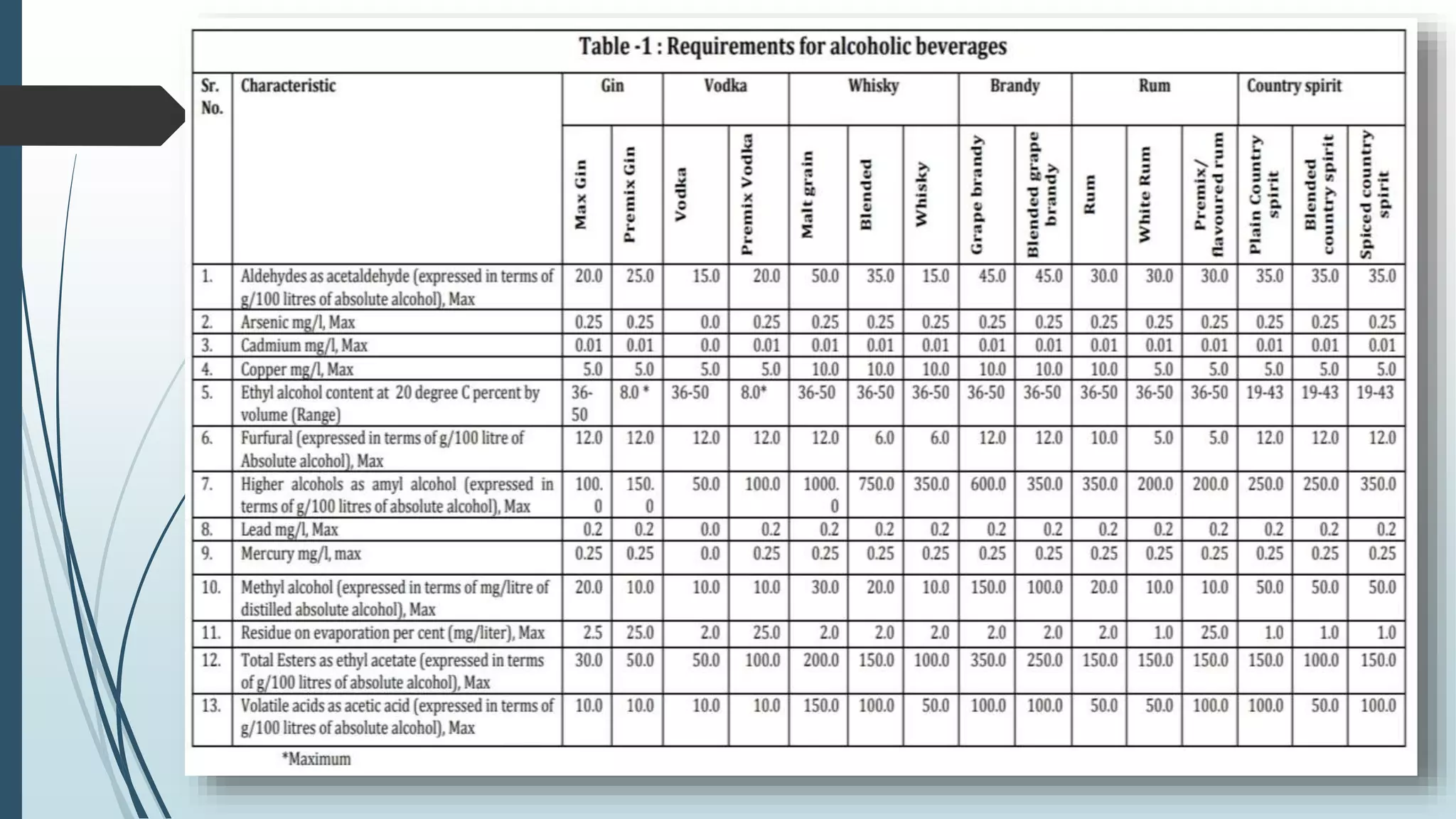
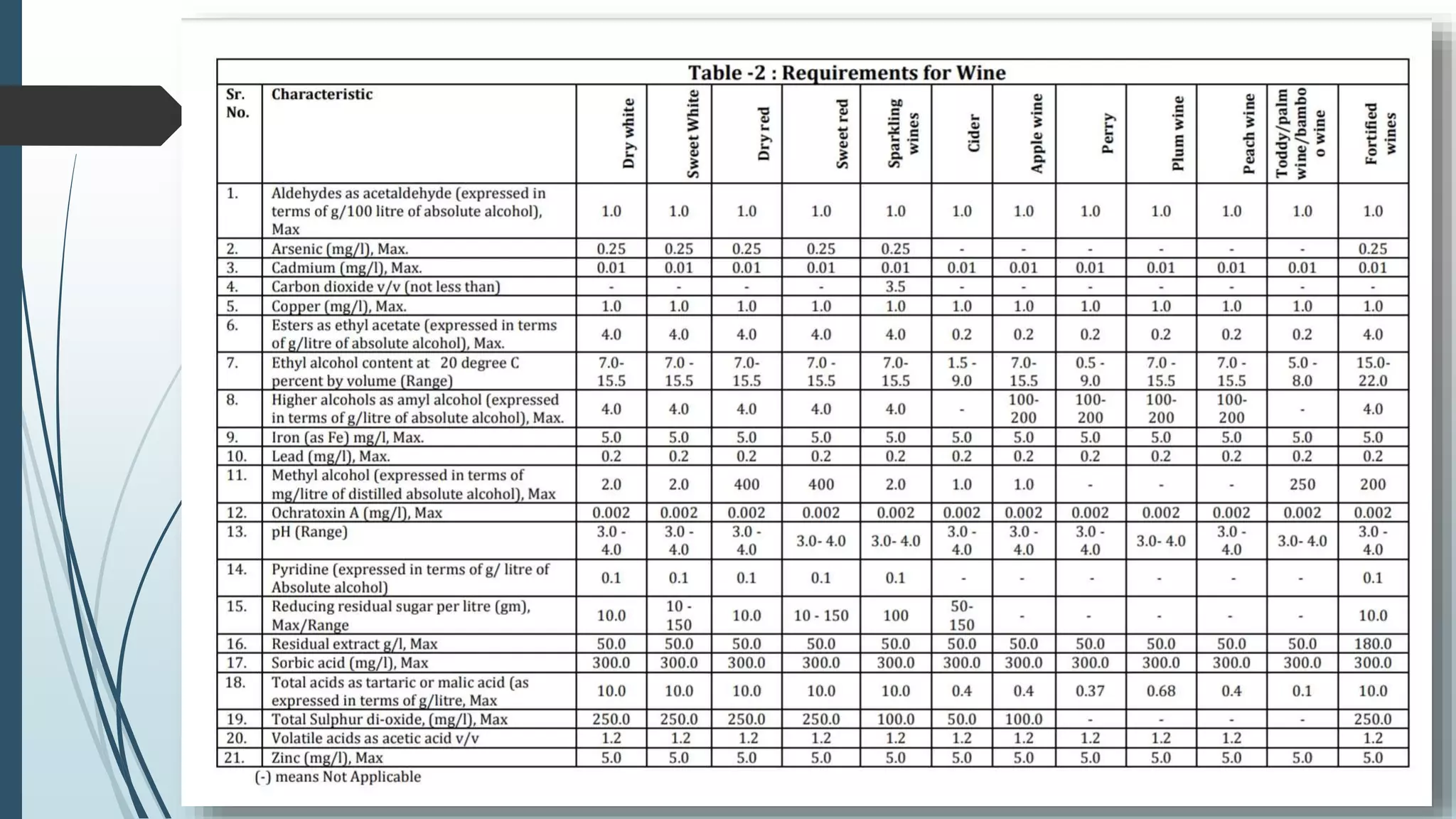
The document outlines quality control and testing parameters in alcoholic beverage production, focusing on both chemical testing and biological identification to maintain product safety and quality standards. It details key tools and processes involved in quality control, including critical control points and various laboratory analysis methods for determining components like ethyl alcohol content, total acidity, and volatile acidity. Additionally, the document provides specific methodologies for testing these parameters, including titration and gas chromatography techniques.