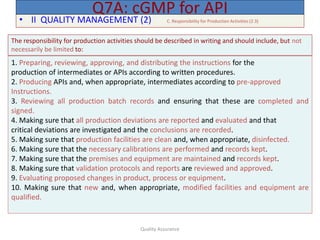
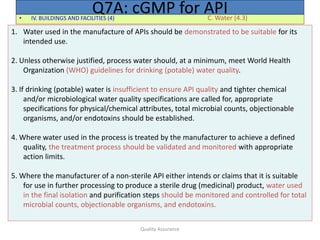
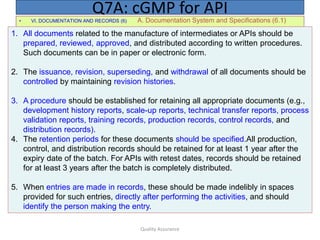
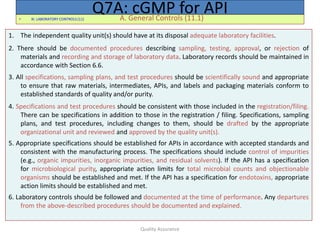
The document outlines quality assurance guidelines for active pharmaceutical ingredients (APIs) as per cGMP regulations. It covers topics such as quality management, personnel qualifications, facility design, utilities, documentation, materials management, production processes, packaging and labeling, storage, validation, change control, and more. The key points are that manufacturers must establish a quality management system involving management participation to ensure APIs meet quality standards, maintain independent quality control units, document all quality-related activities, and conduct periodic reviews and audits.


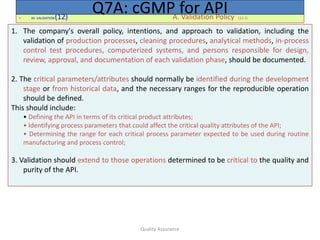
![Quality Assurance
Q7A: cGMP
for Active Pharmaceutical Ingredient (API)
1. Quality should be the responsibility of all persons involved in mfg.
2. Each manufacturer should establish, document, and implement an effective
system for managing quality that involves the active participation of management.
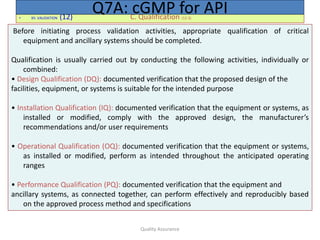
3. The system for managing quality should encompass the organizational structure,
procedures, processes and resources, as well as activities to ensure confidence
that the API will meet its intended specifications for quality and purity. All quality-
related activities should be defined and documented.
4. There should be a quality unit(s) that is independent of production and that fulfills
both QA and QC responsibilities.
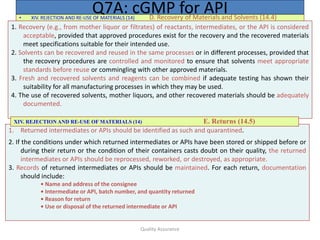
5. The persons authorized to release intermediates and APIs should be specified. [It
should be the QA person (Ref. WHO-TRS-908)].
6. All quality-related activities should be recorded at the time performed.
7. Any deviation from established procedures should be documented and explained.
Critical deviations should be investigated, and documented.
8. No materials should be released or used before the satisfactory completion of
evaluation by the quality unit(s).
9. Procedures should exist for notifying responsible management in a timely manner
of regulatory inspections, serious GMP deficiencies, product defects and related
actions (e.g., quality-related complaints, recalls, and regulatory actions).
II QUALITY MANAGEMENT (2) A. Principles (2.1)](https://image.slidesharecdn.com/q7a-150211095516-conversion-gate01/85/Q7-a-3-320.jpg)