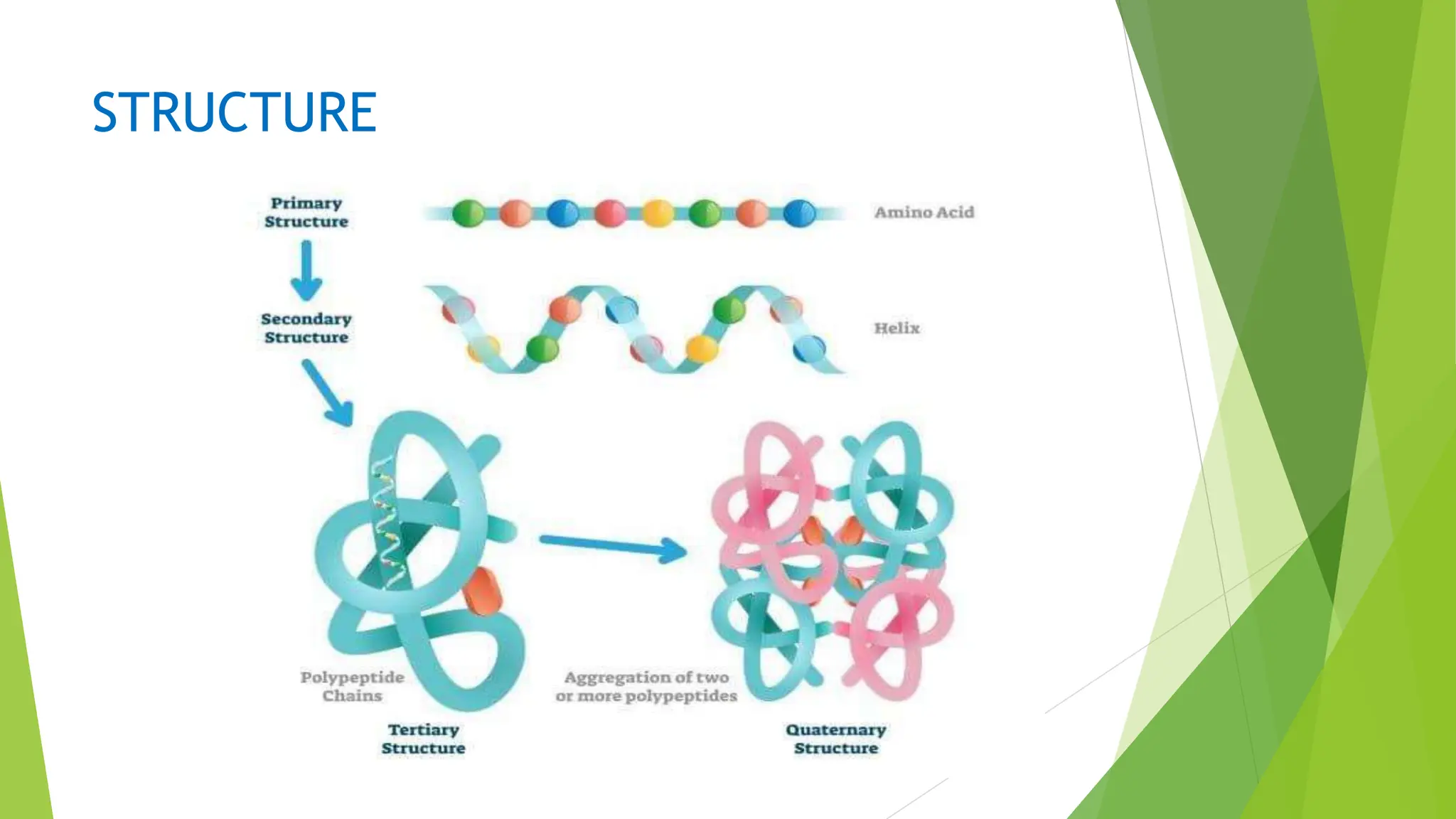
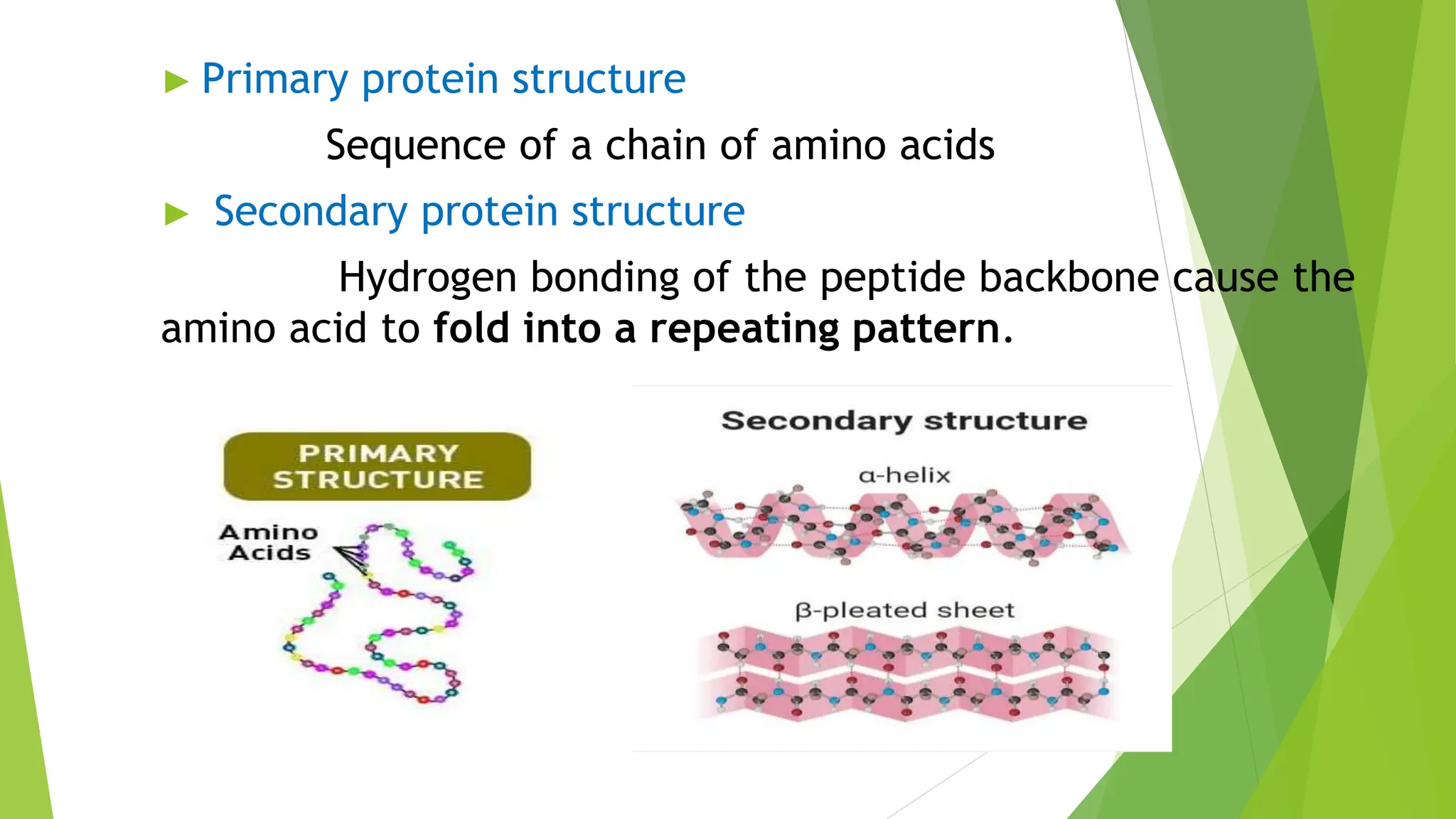
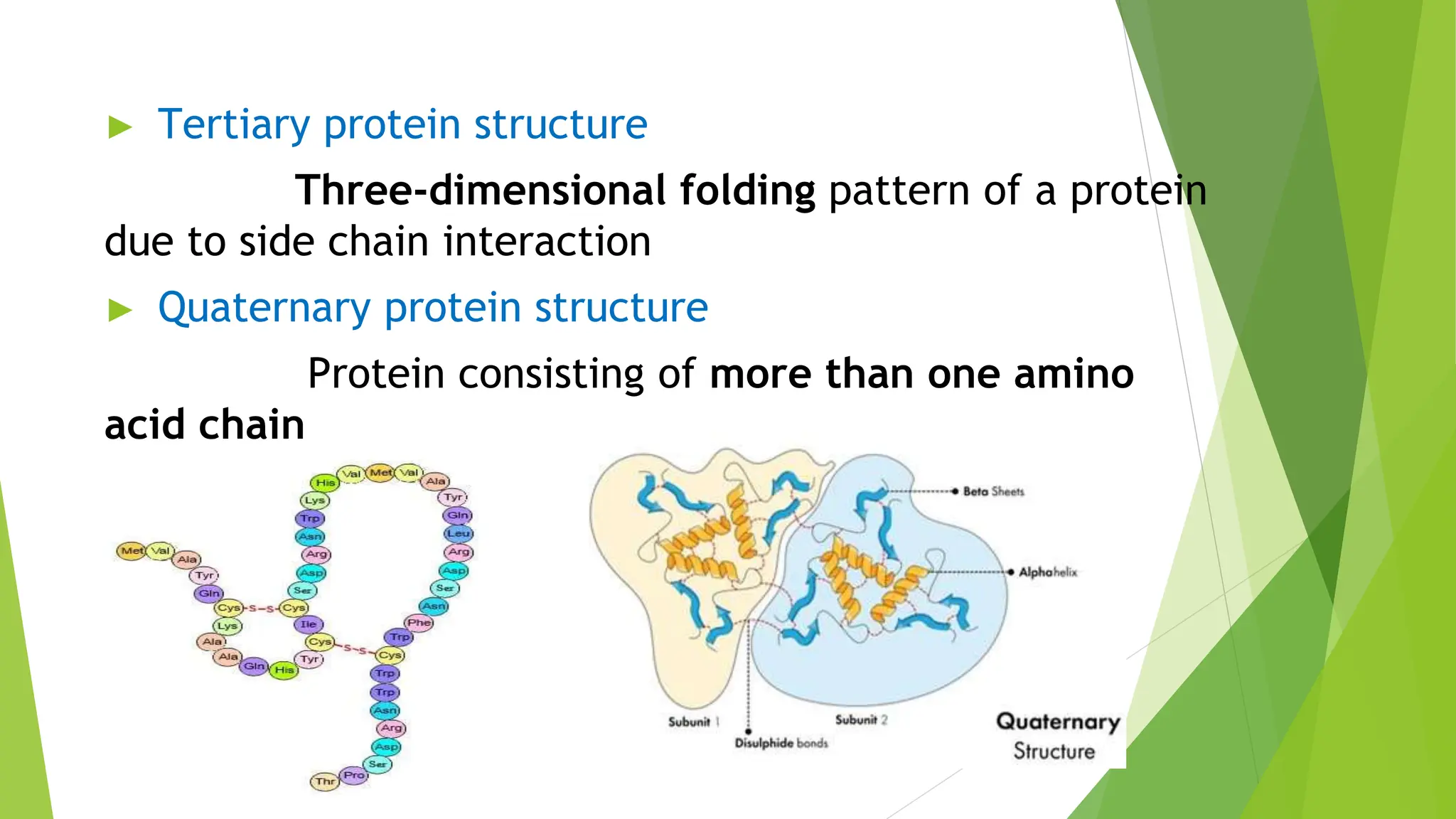
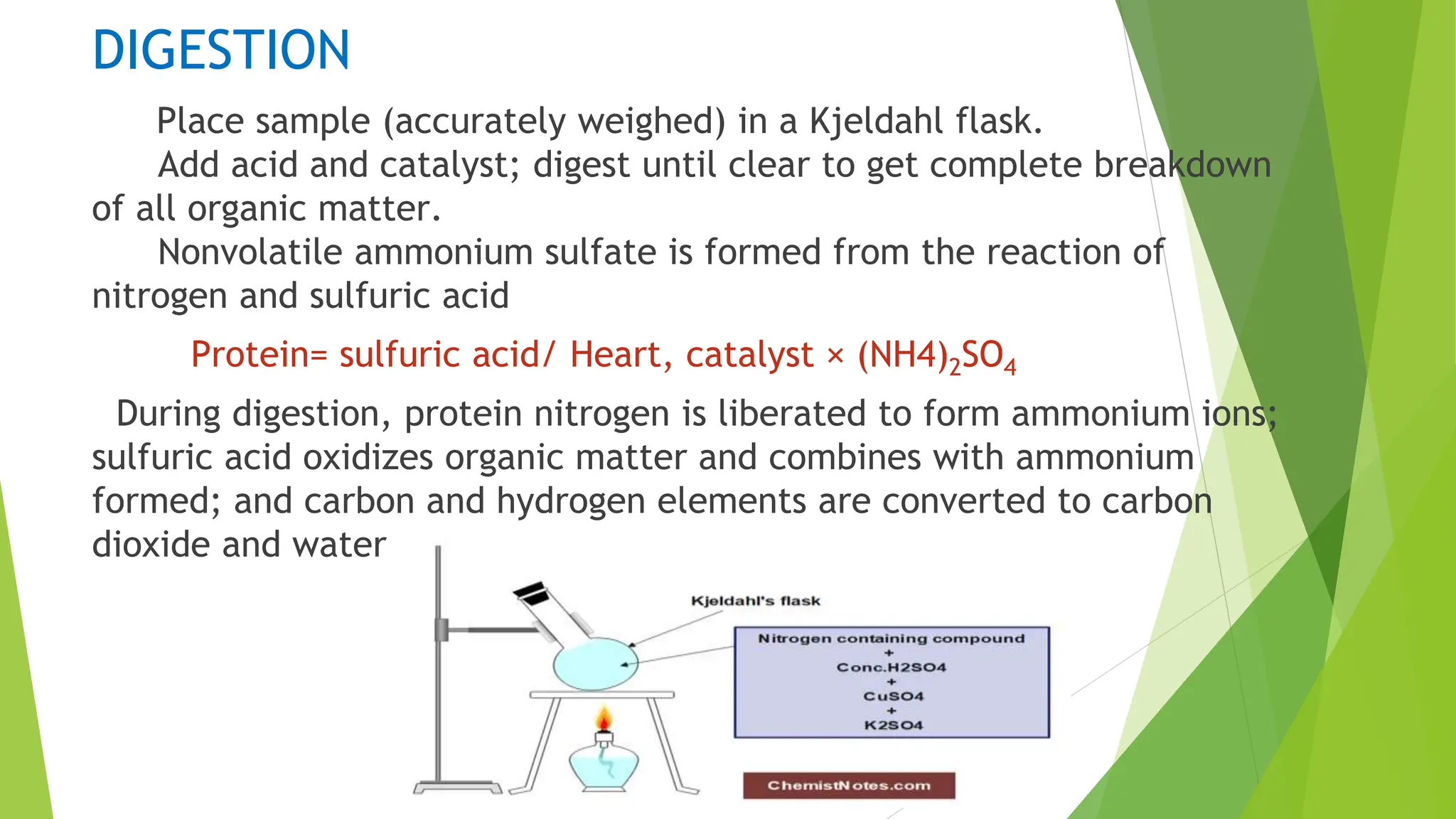
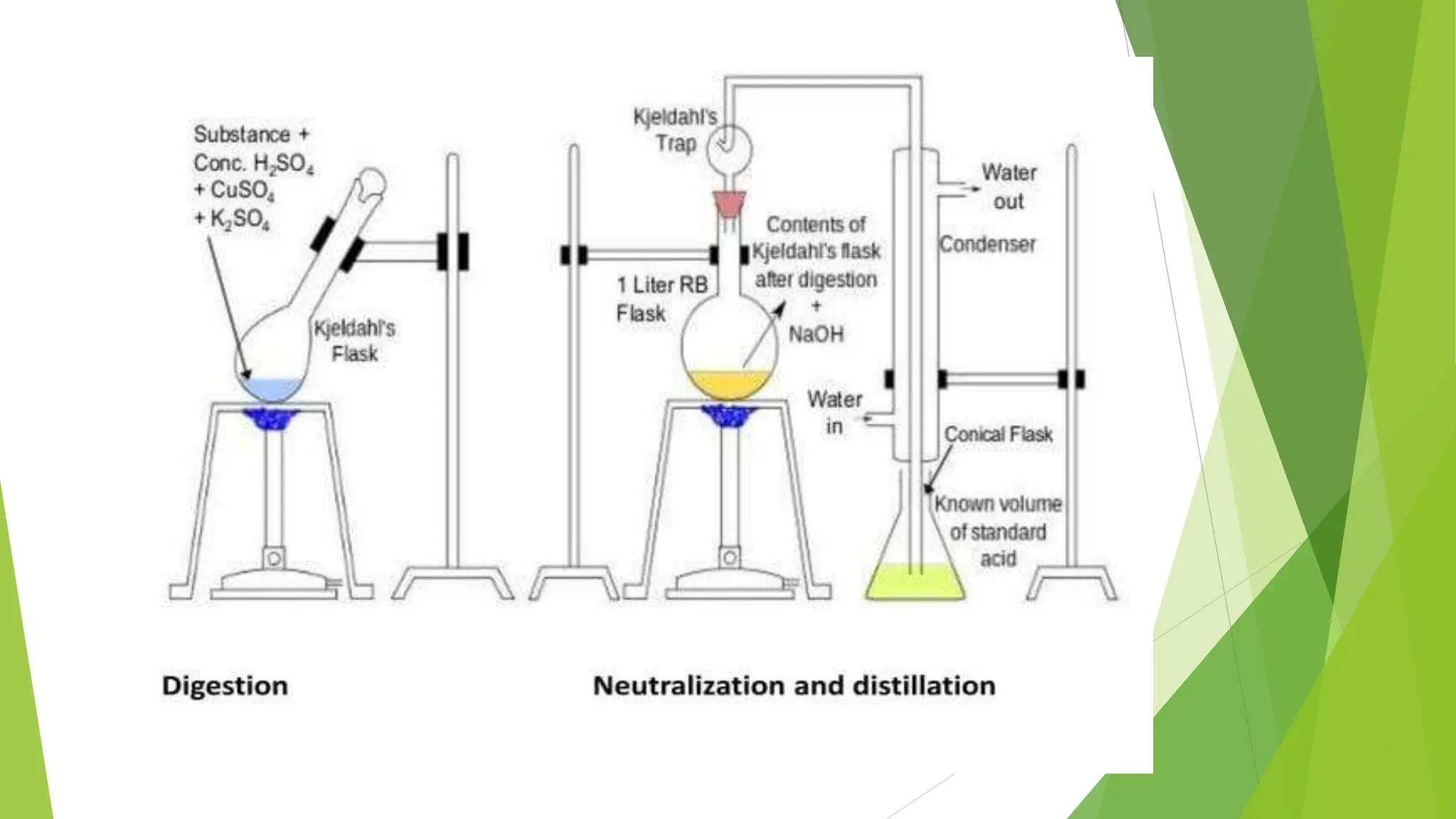
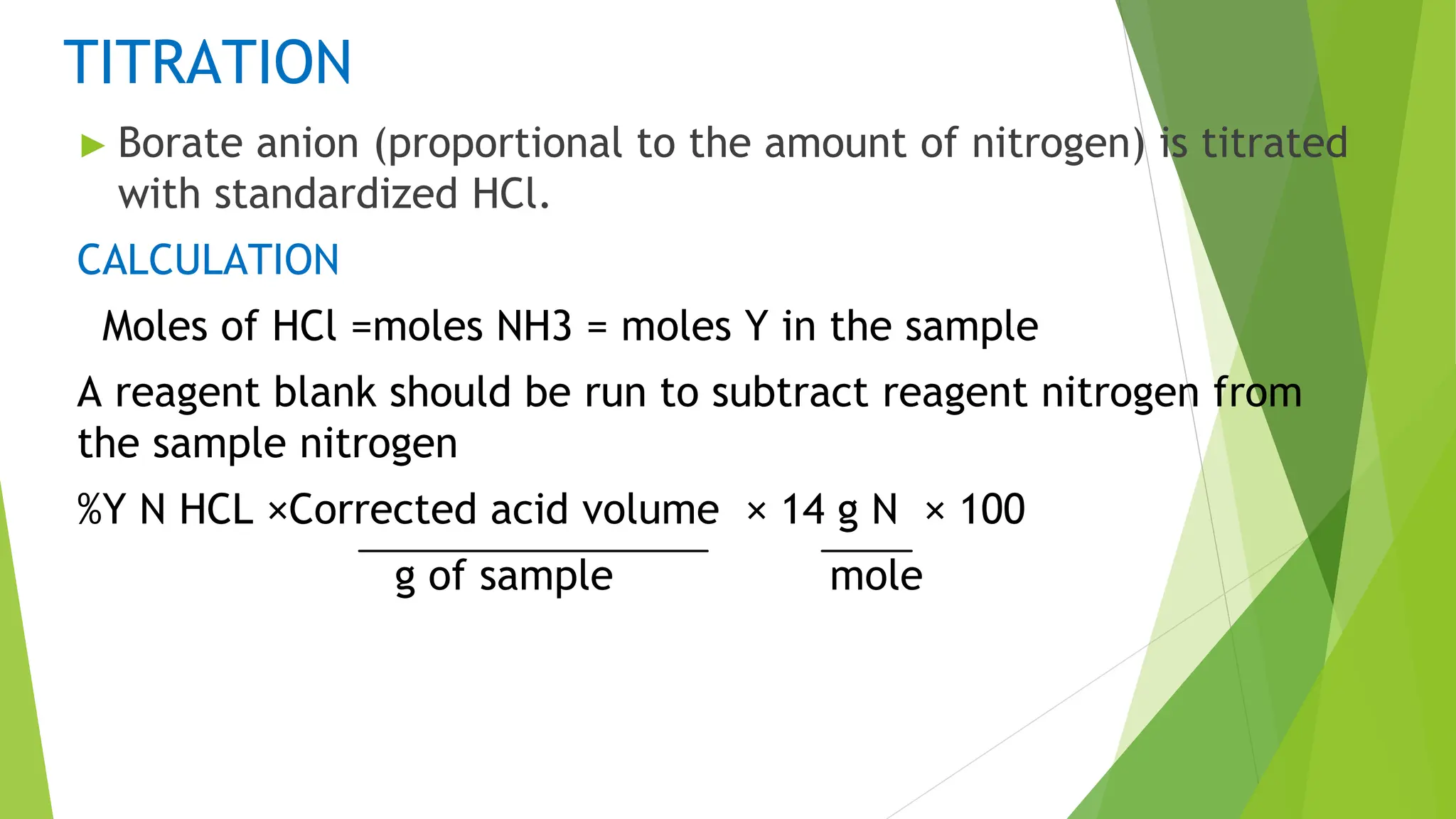
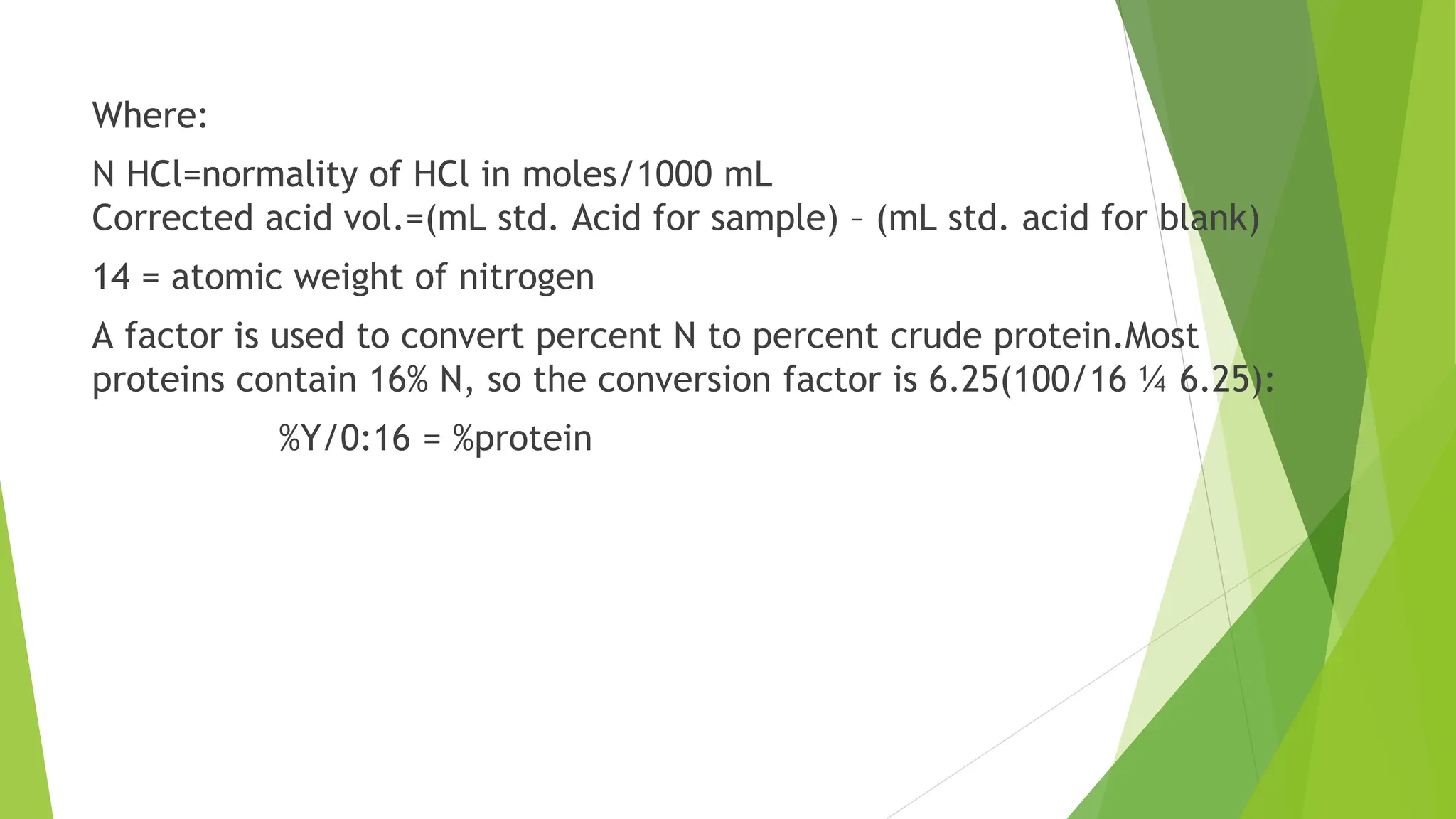
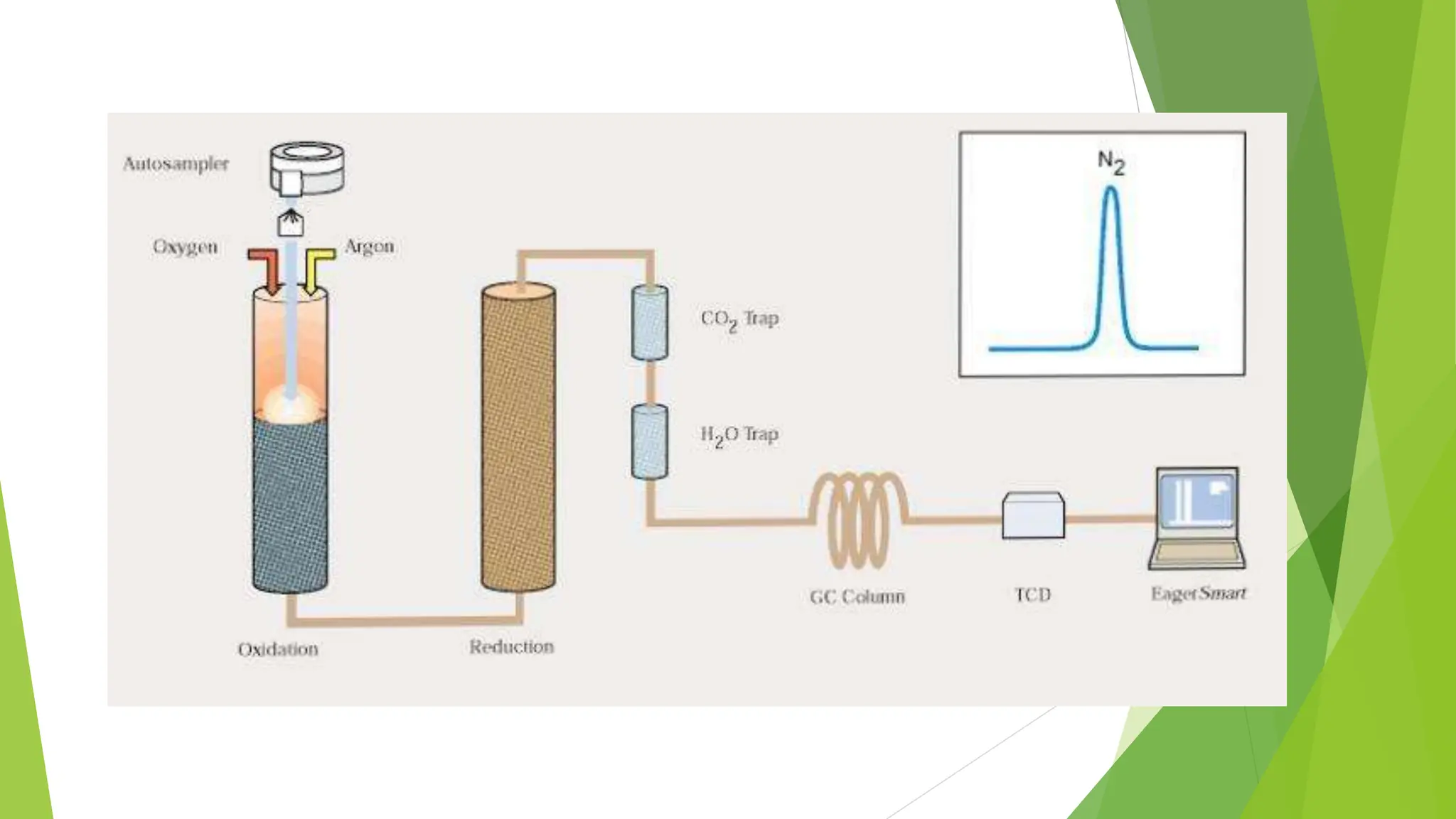
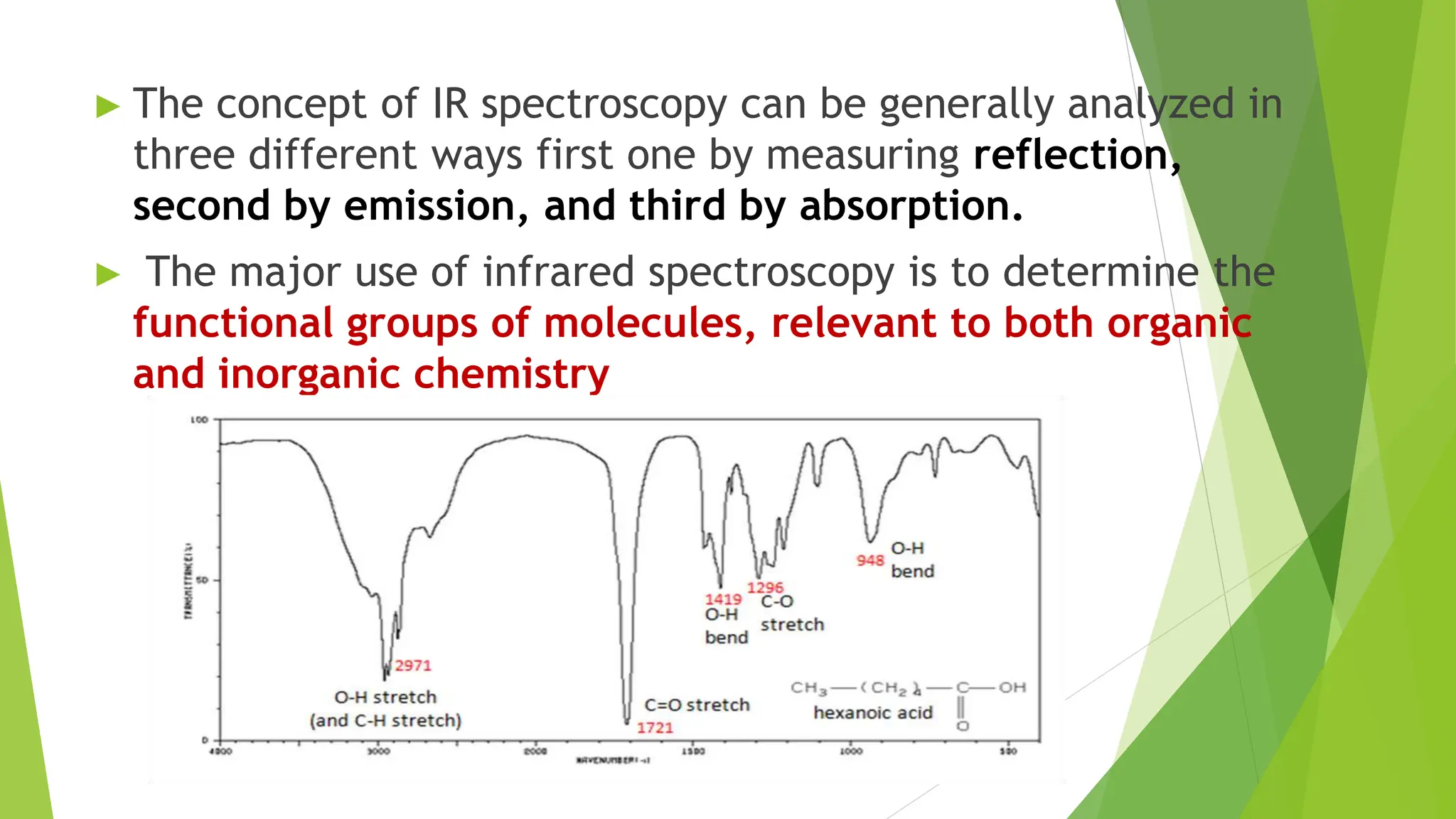
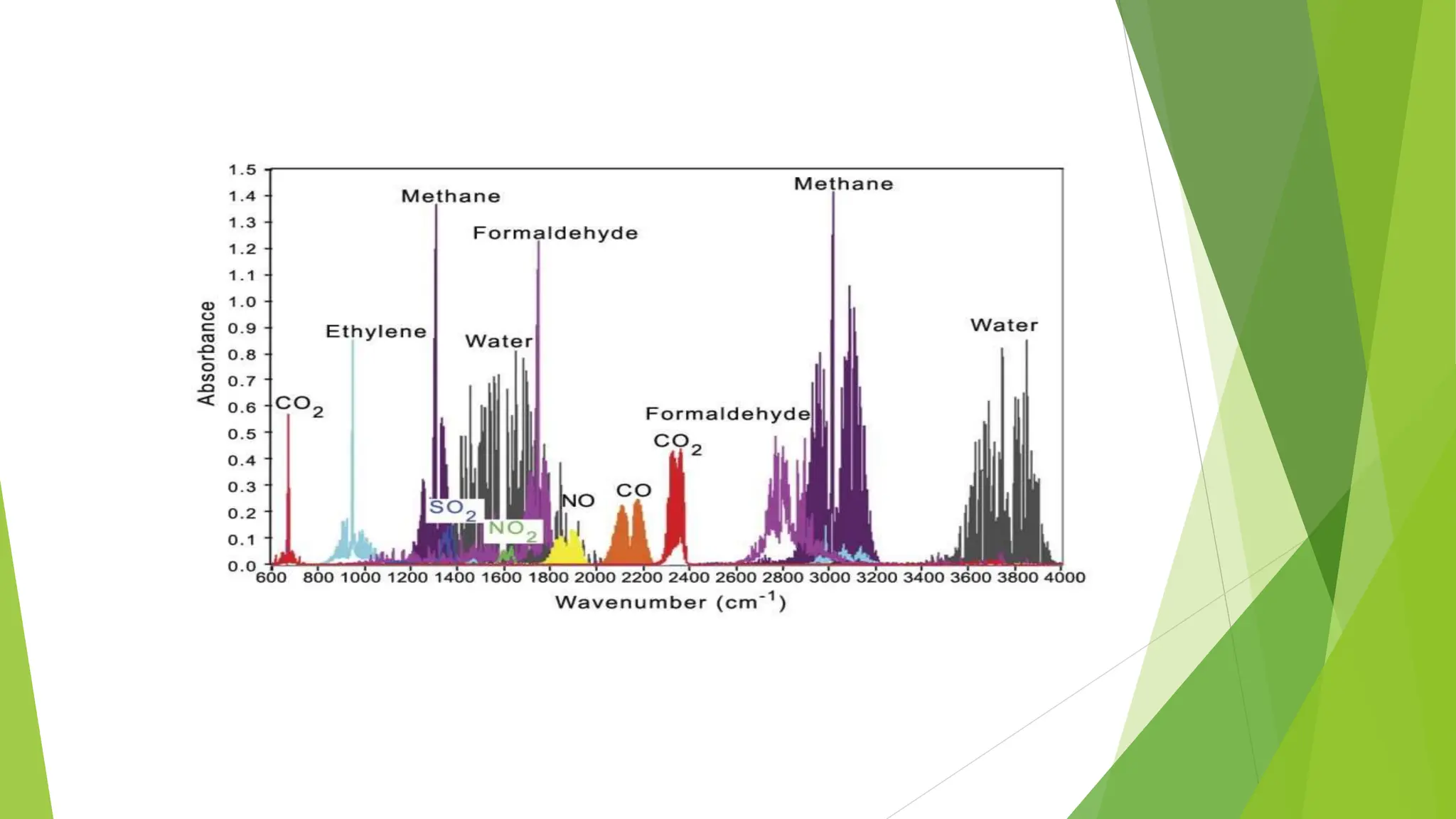
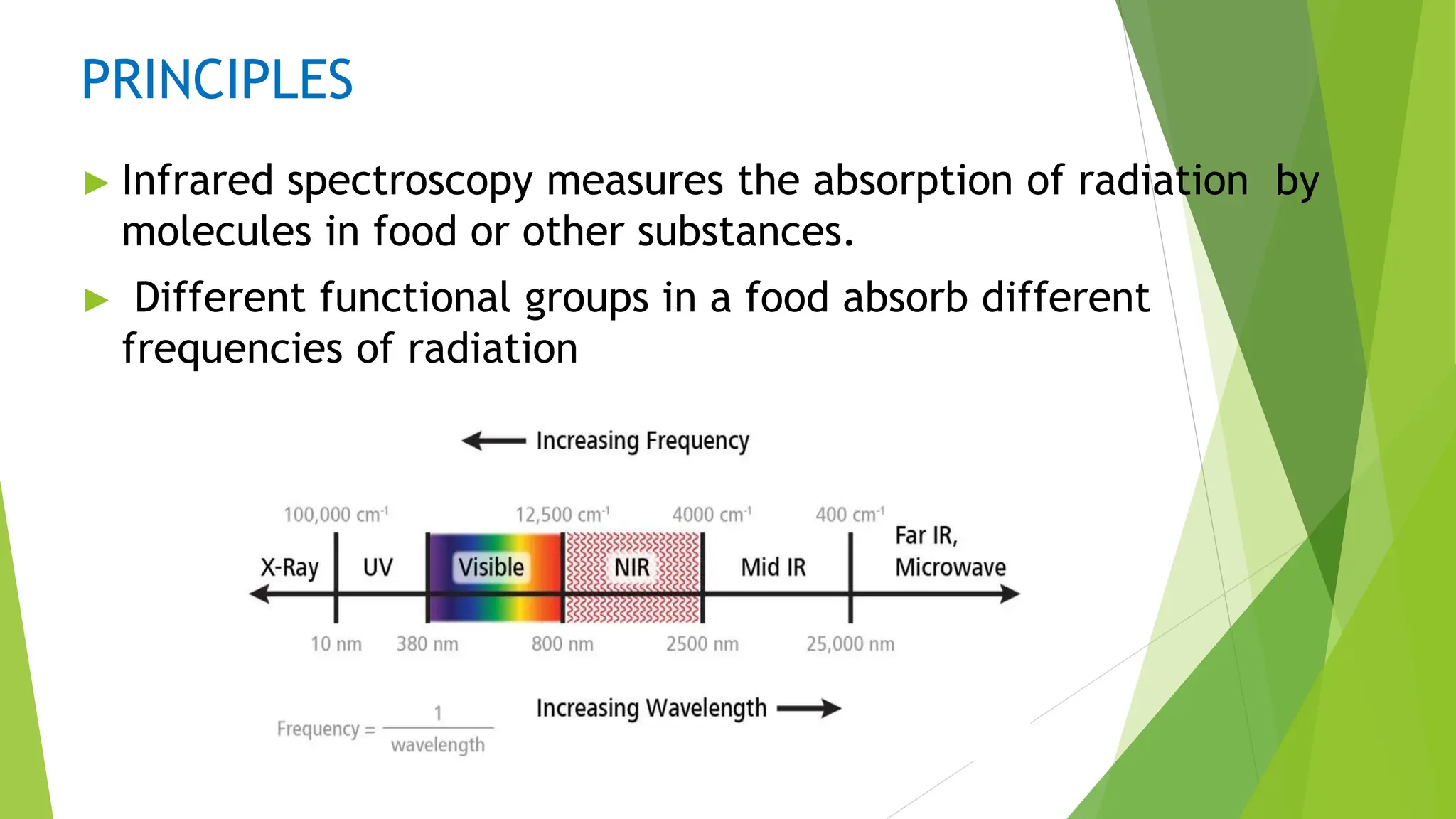
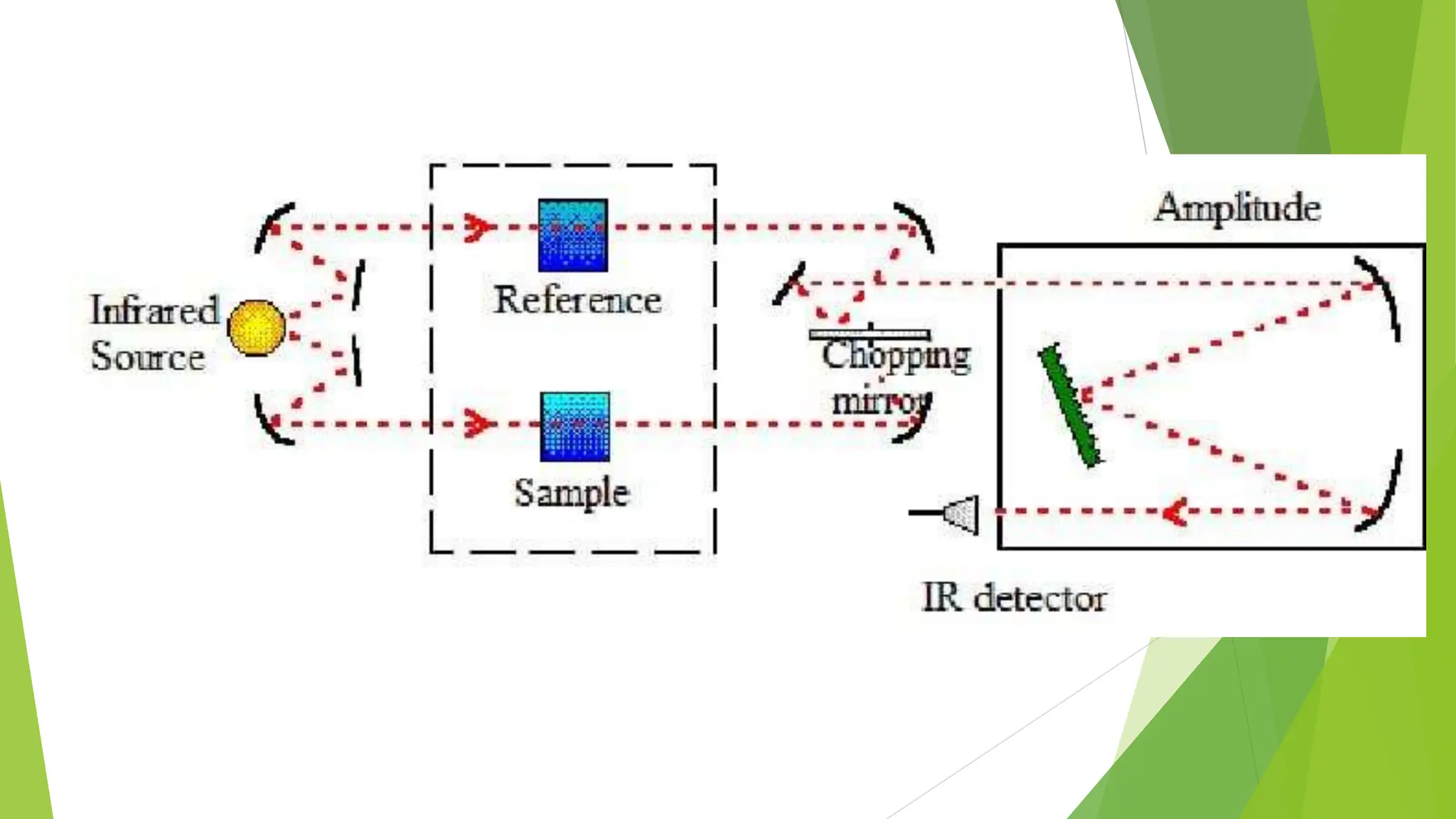
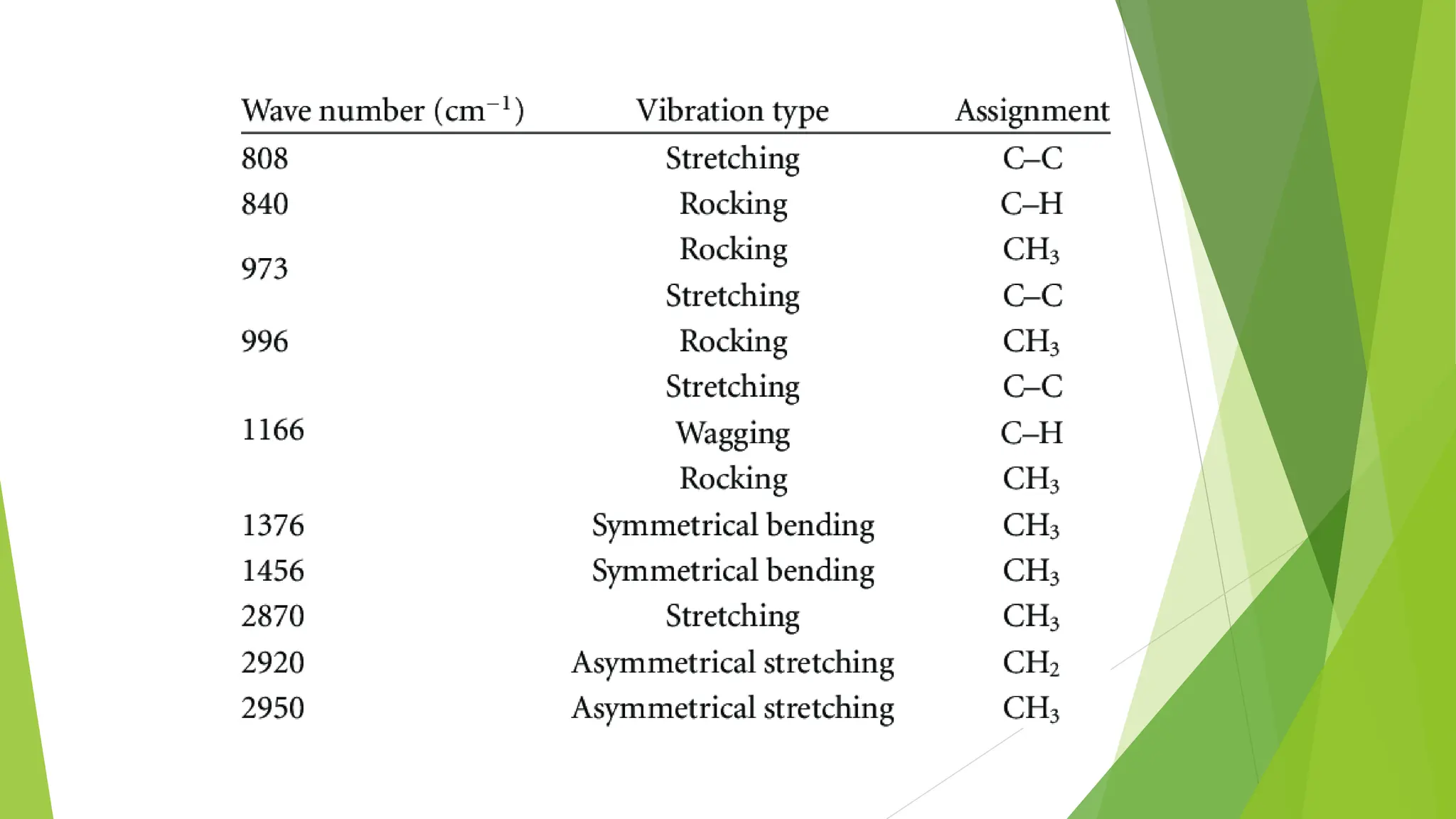
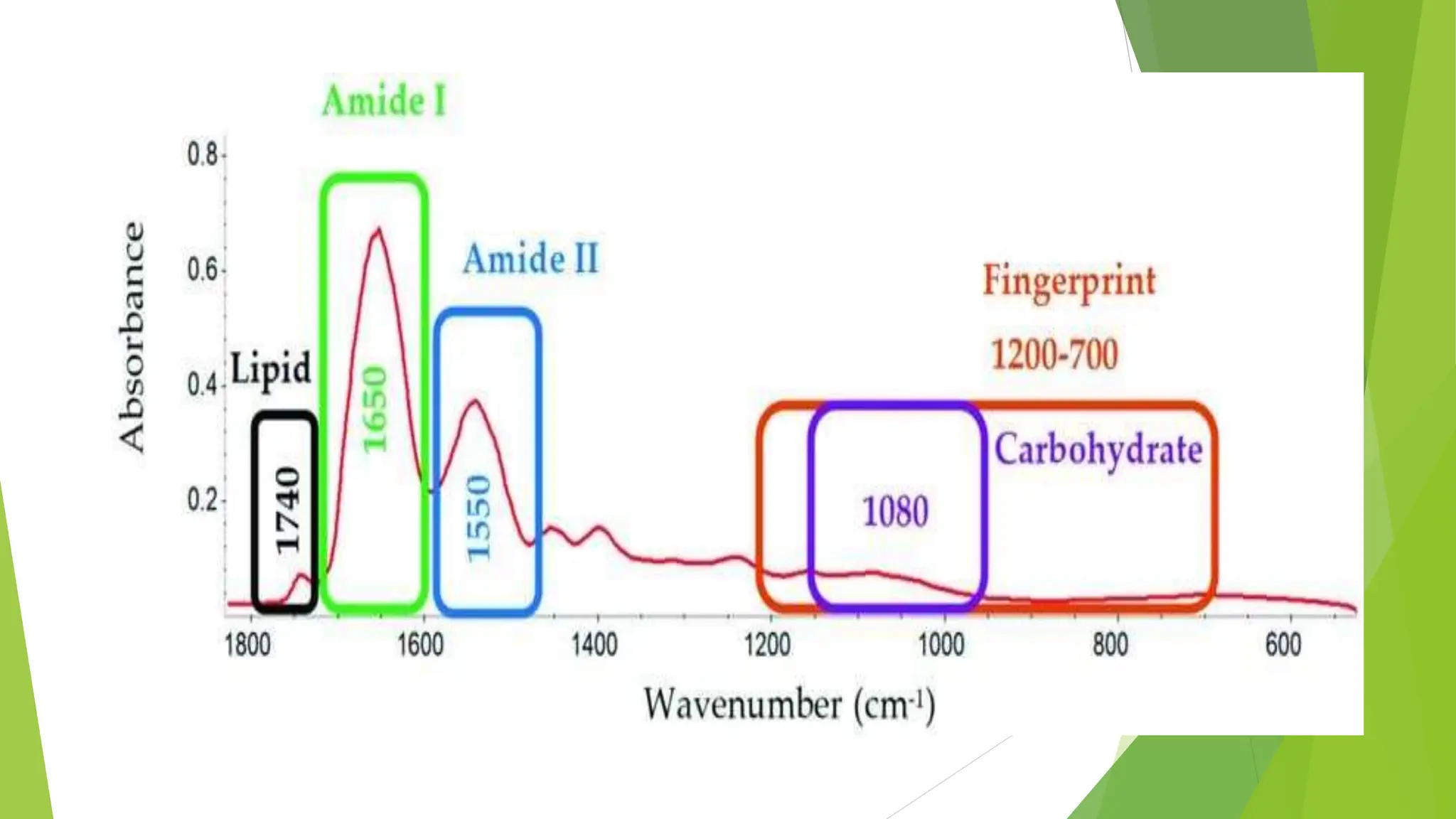
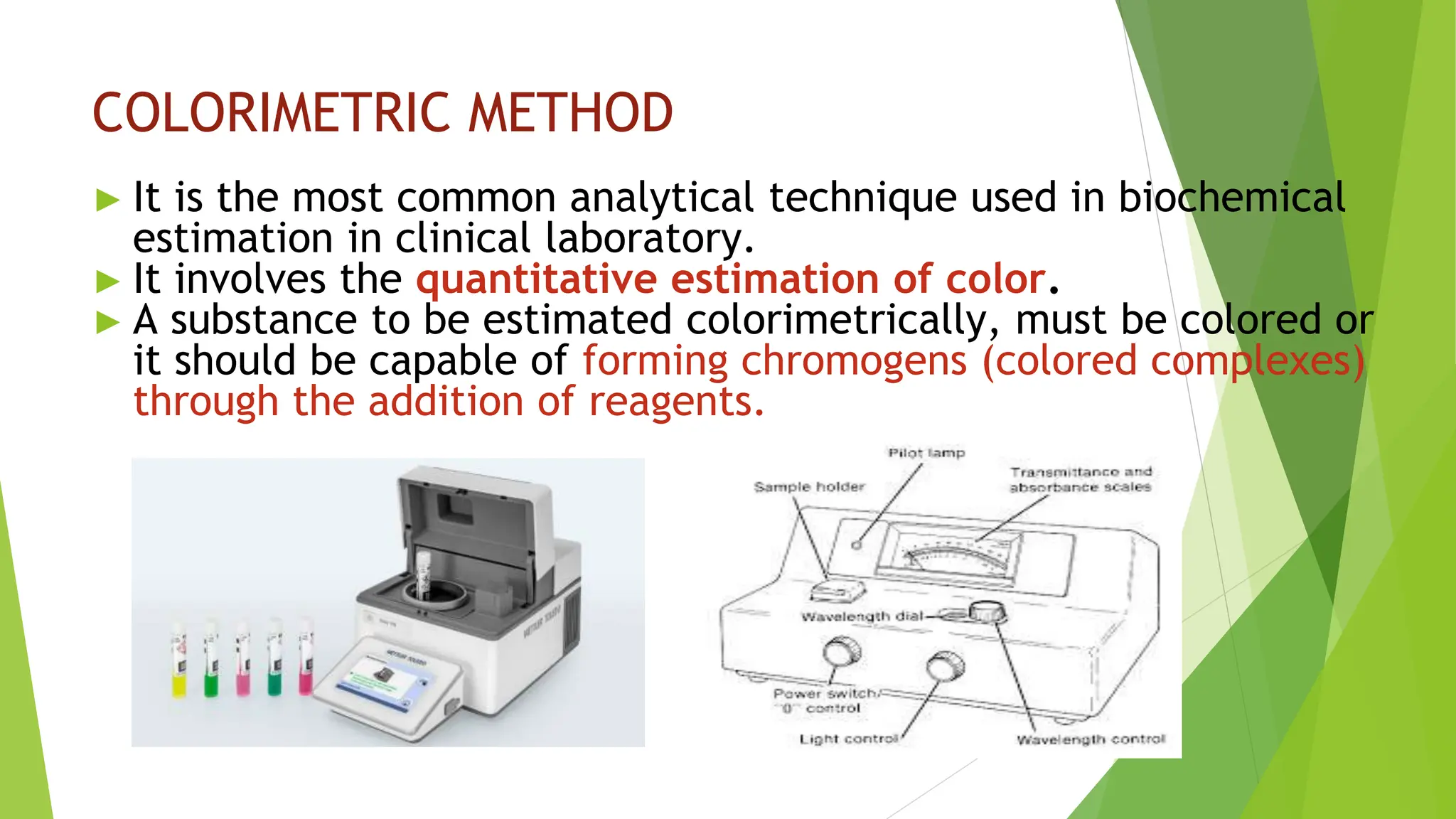
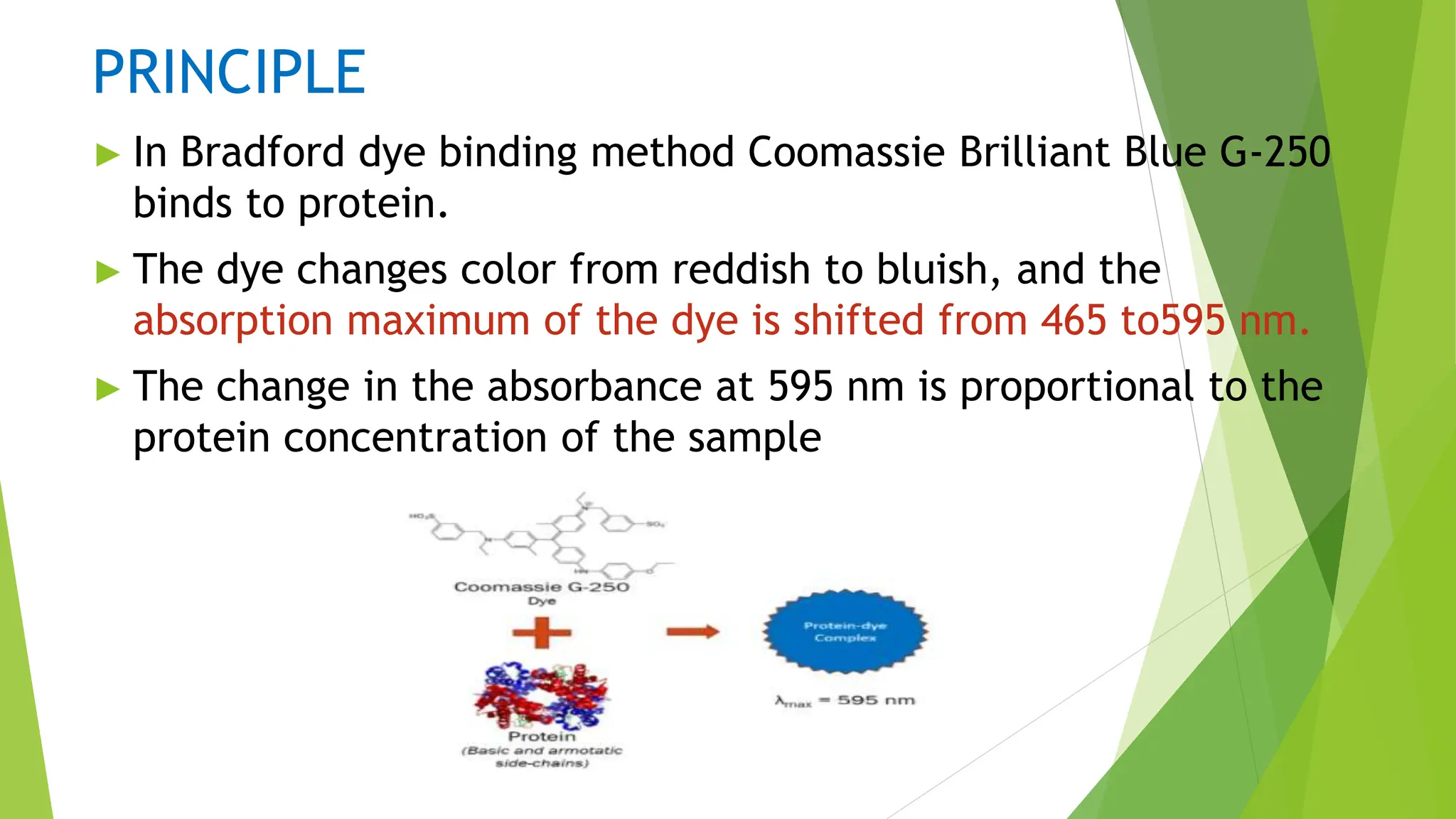
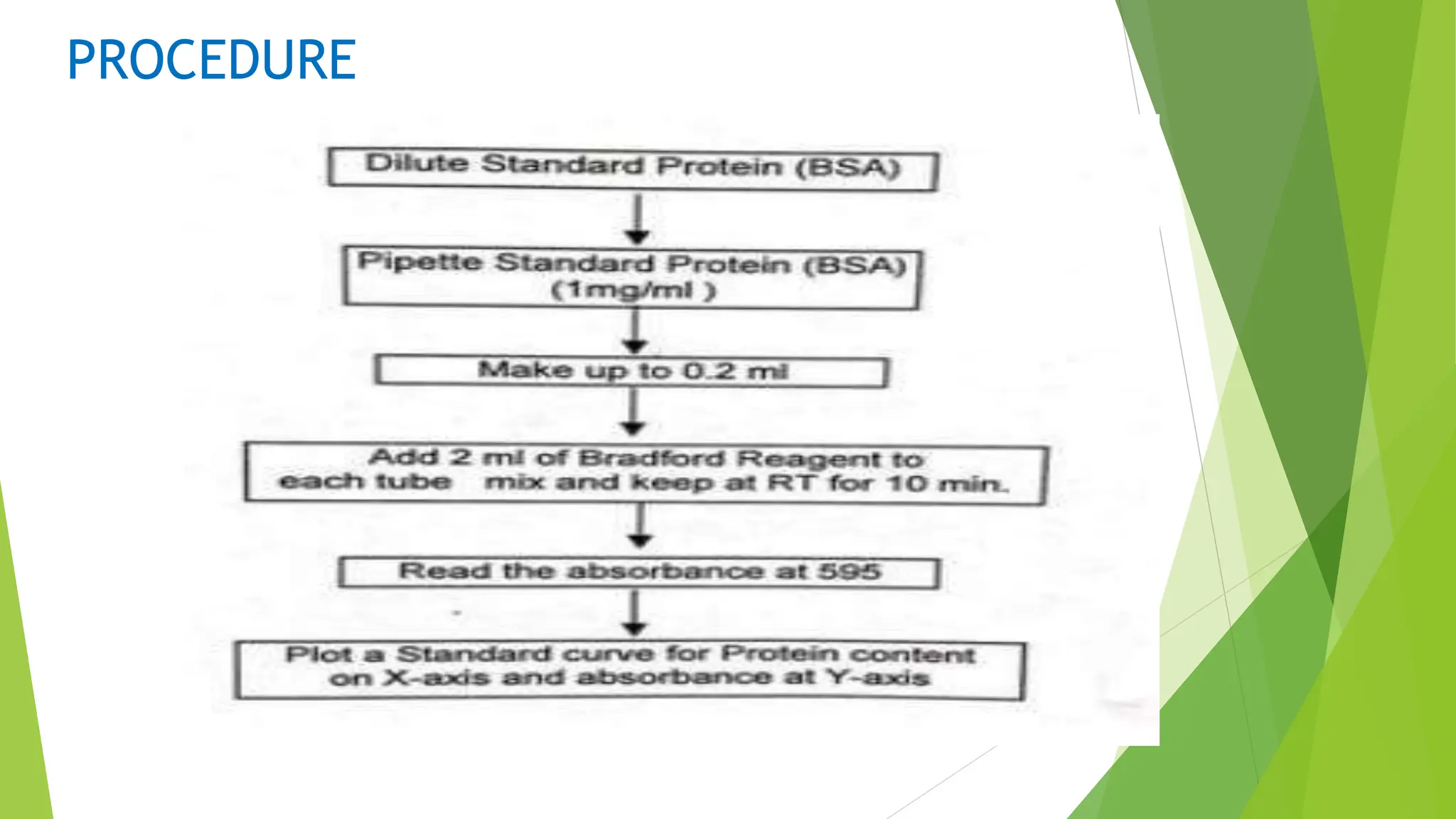
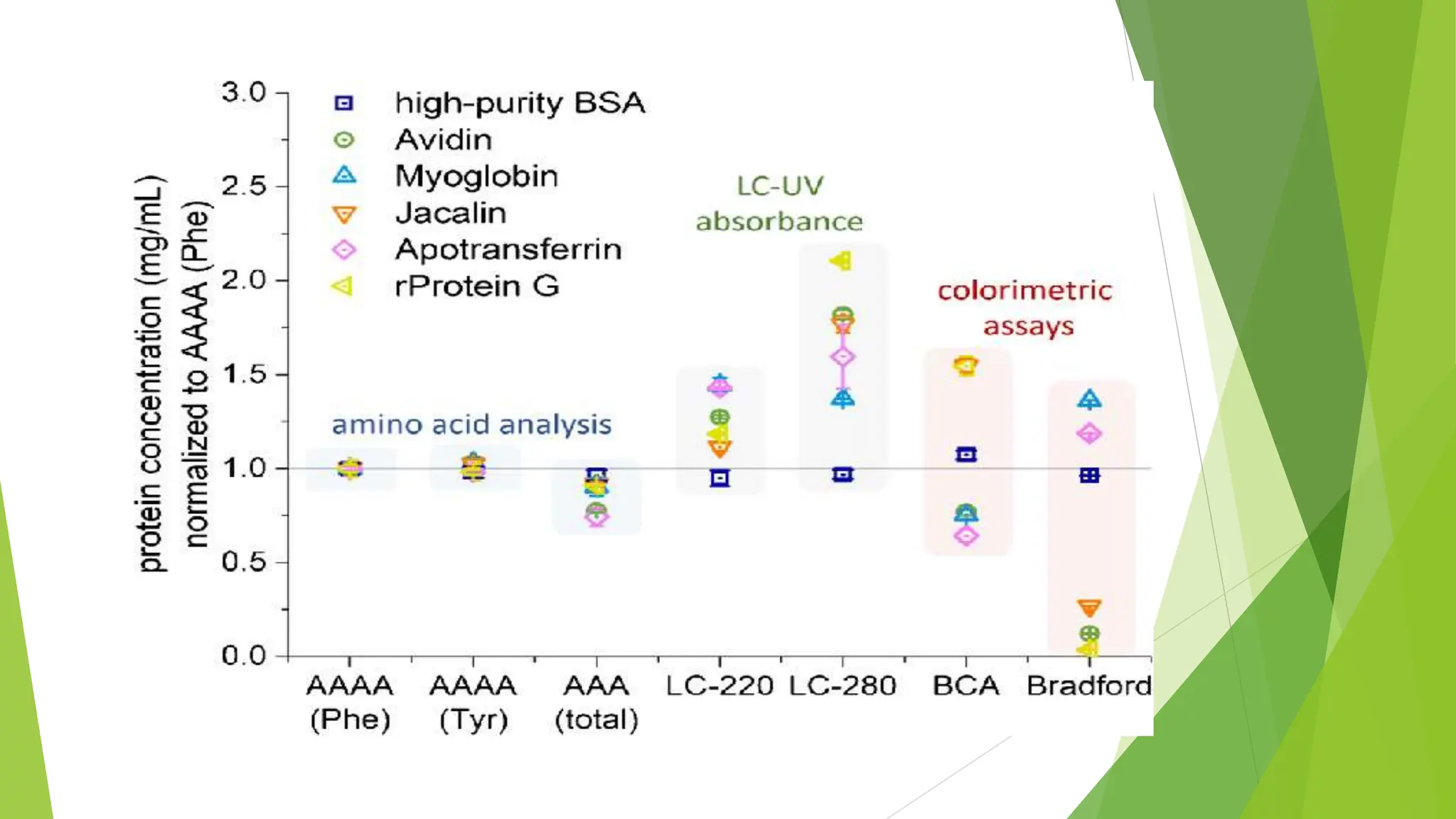
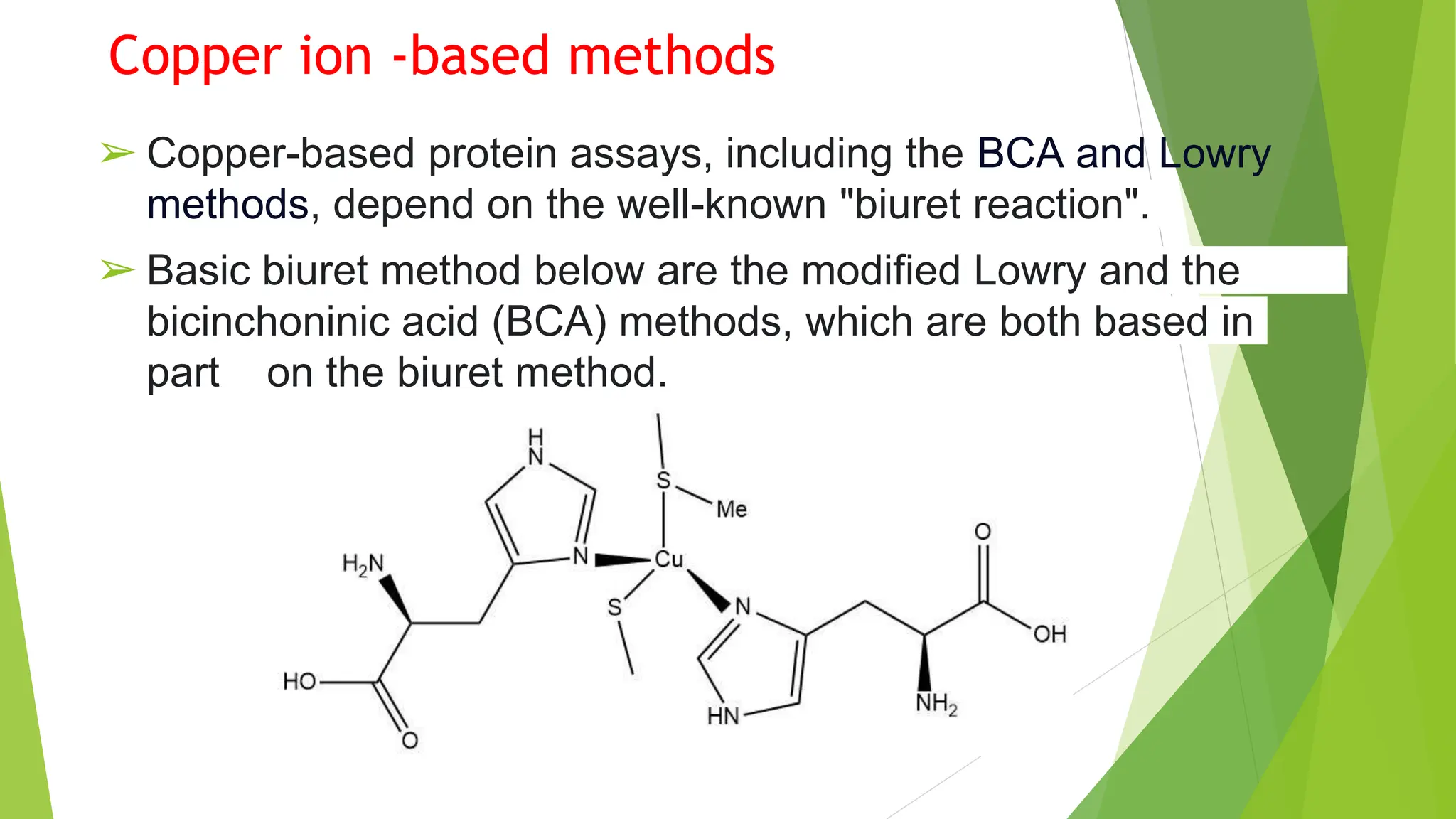
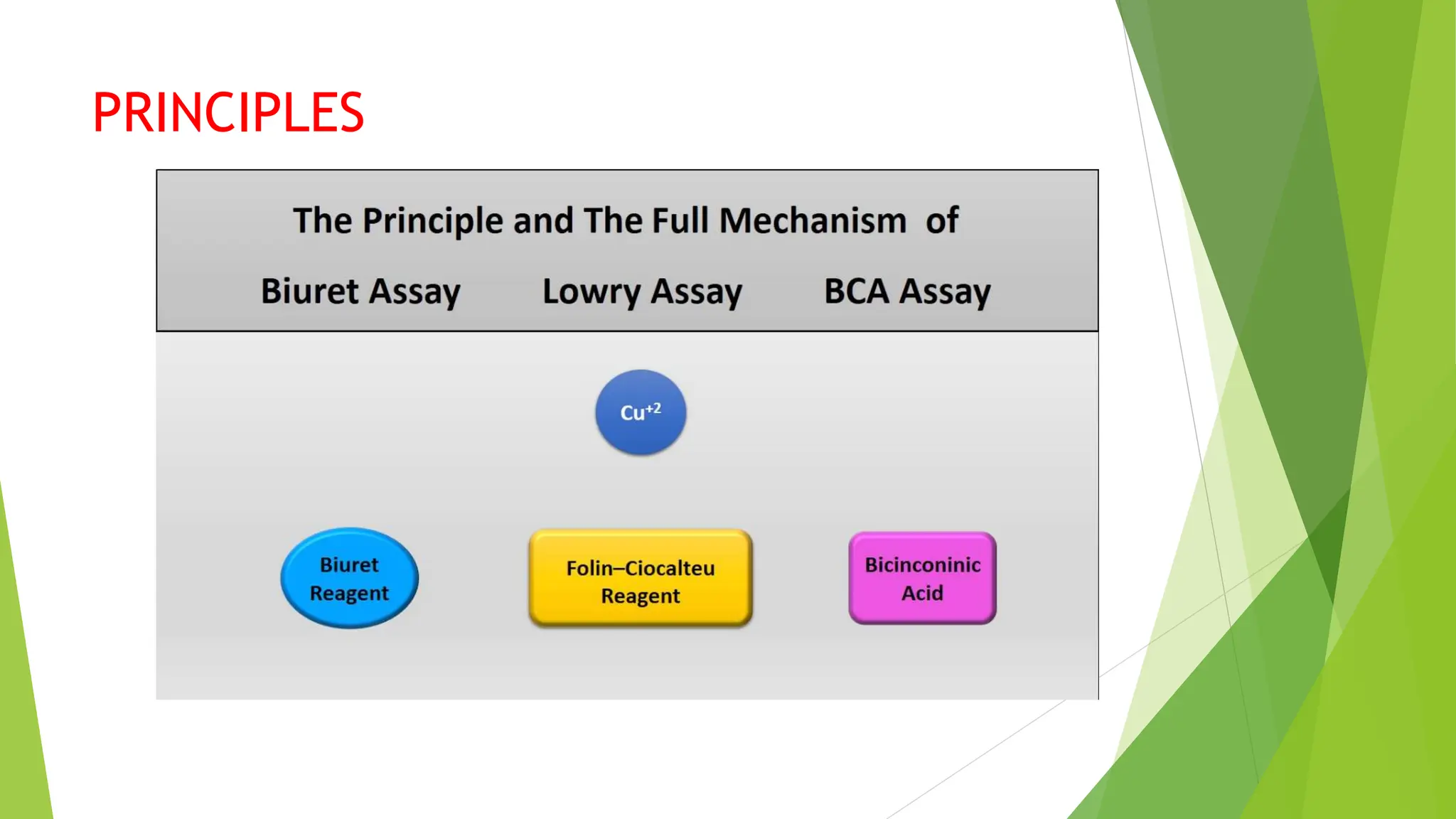
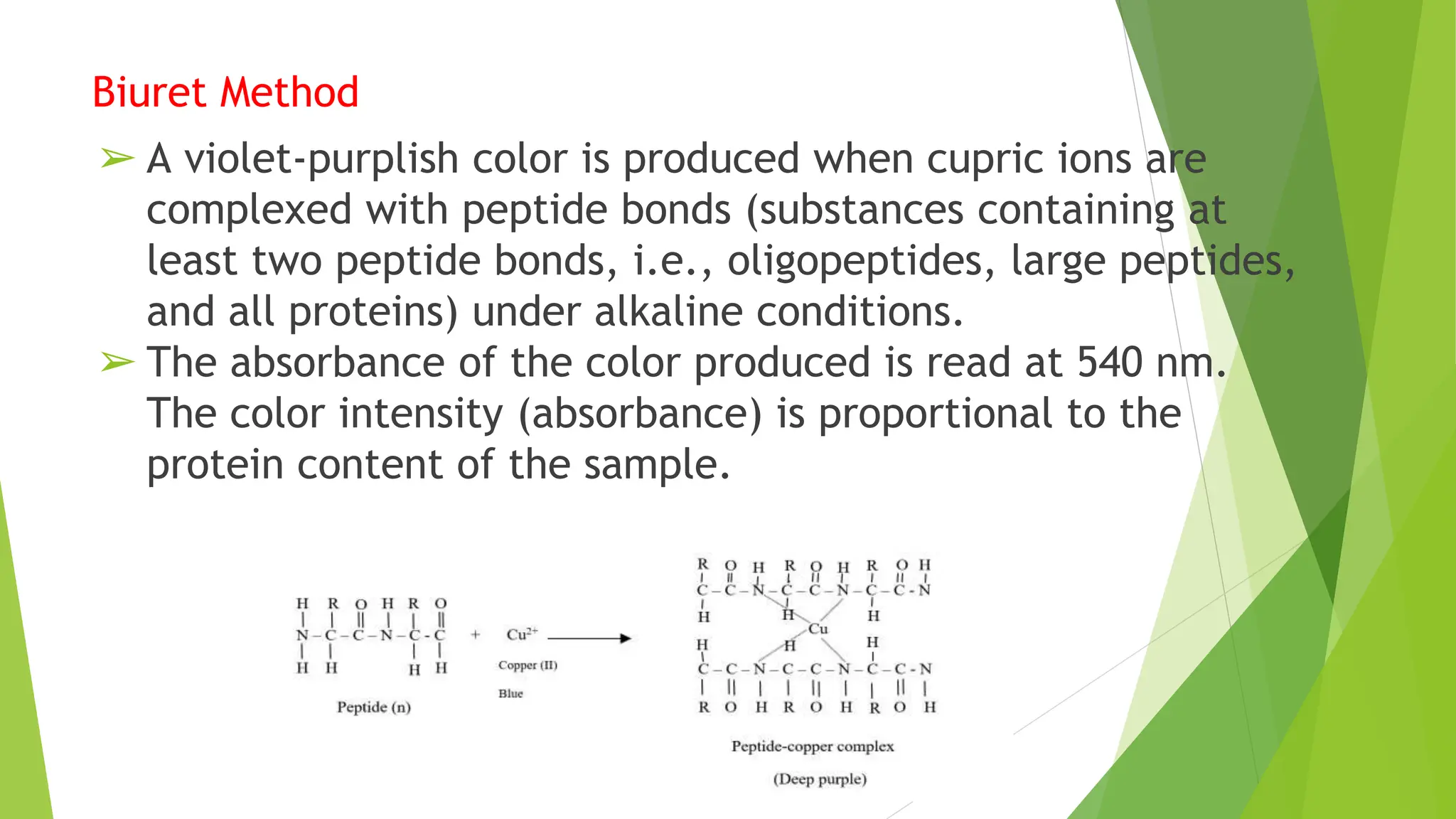
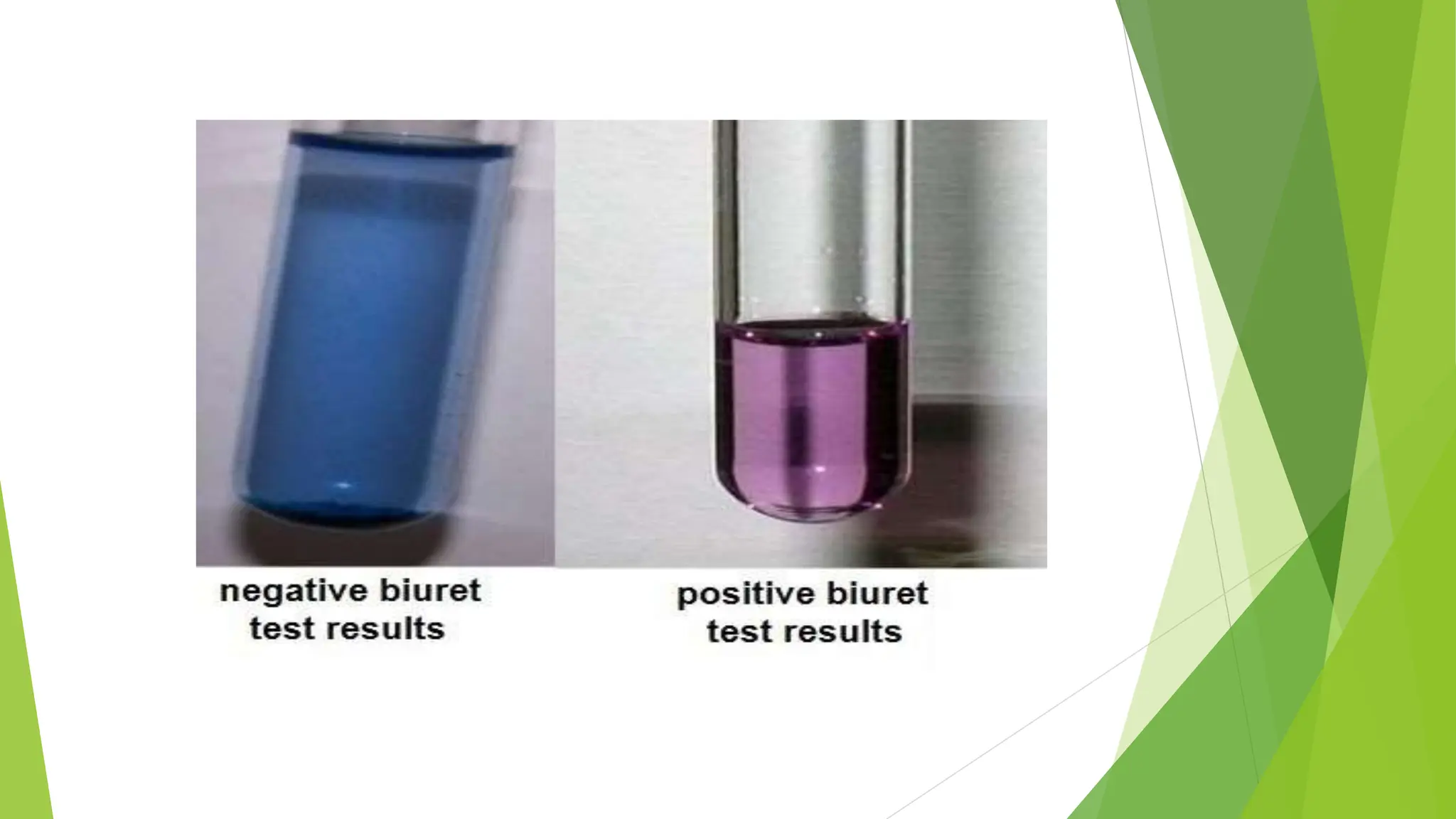
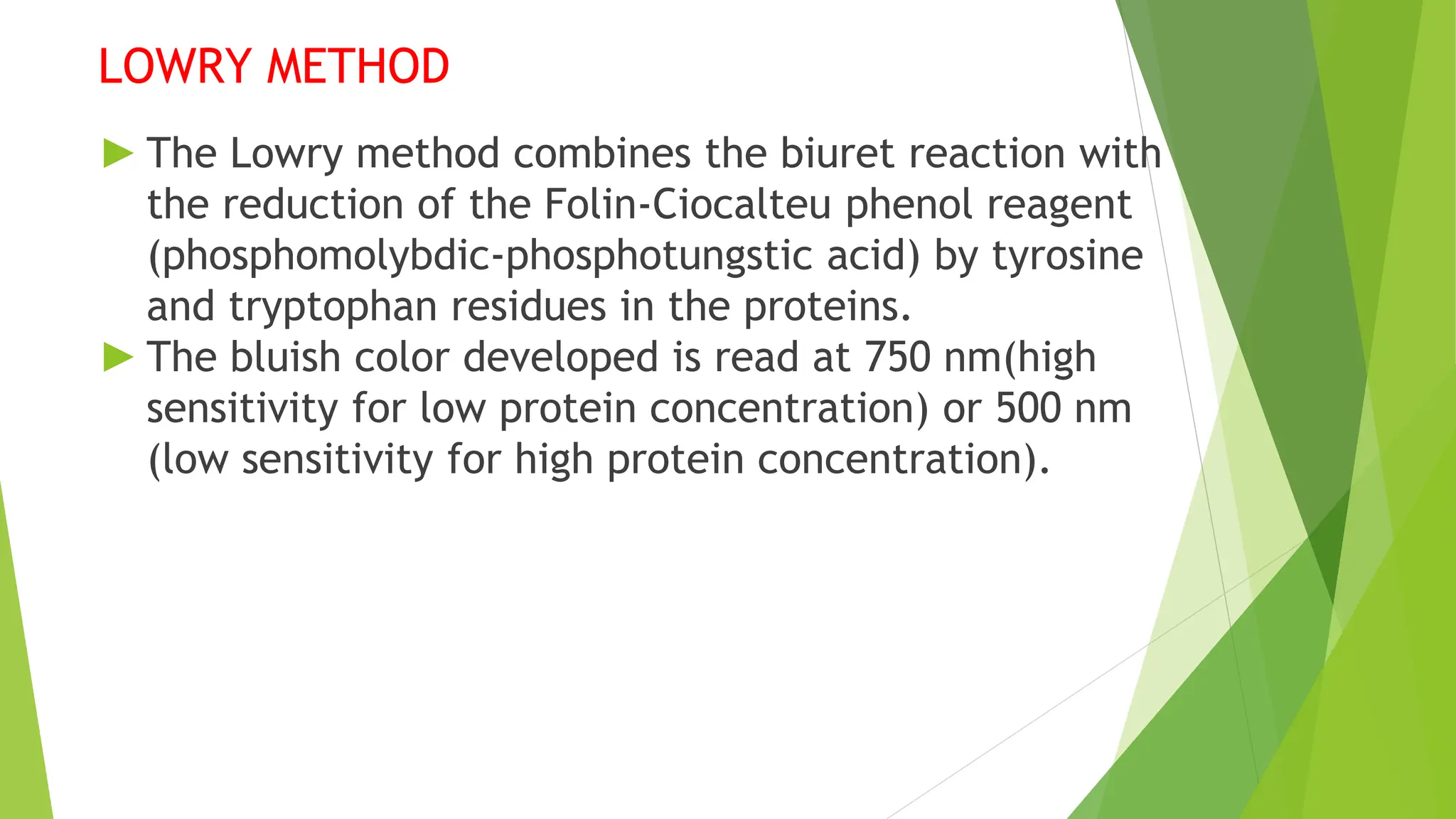
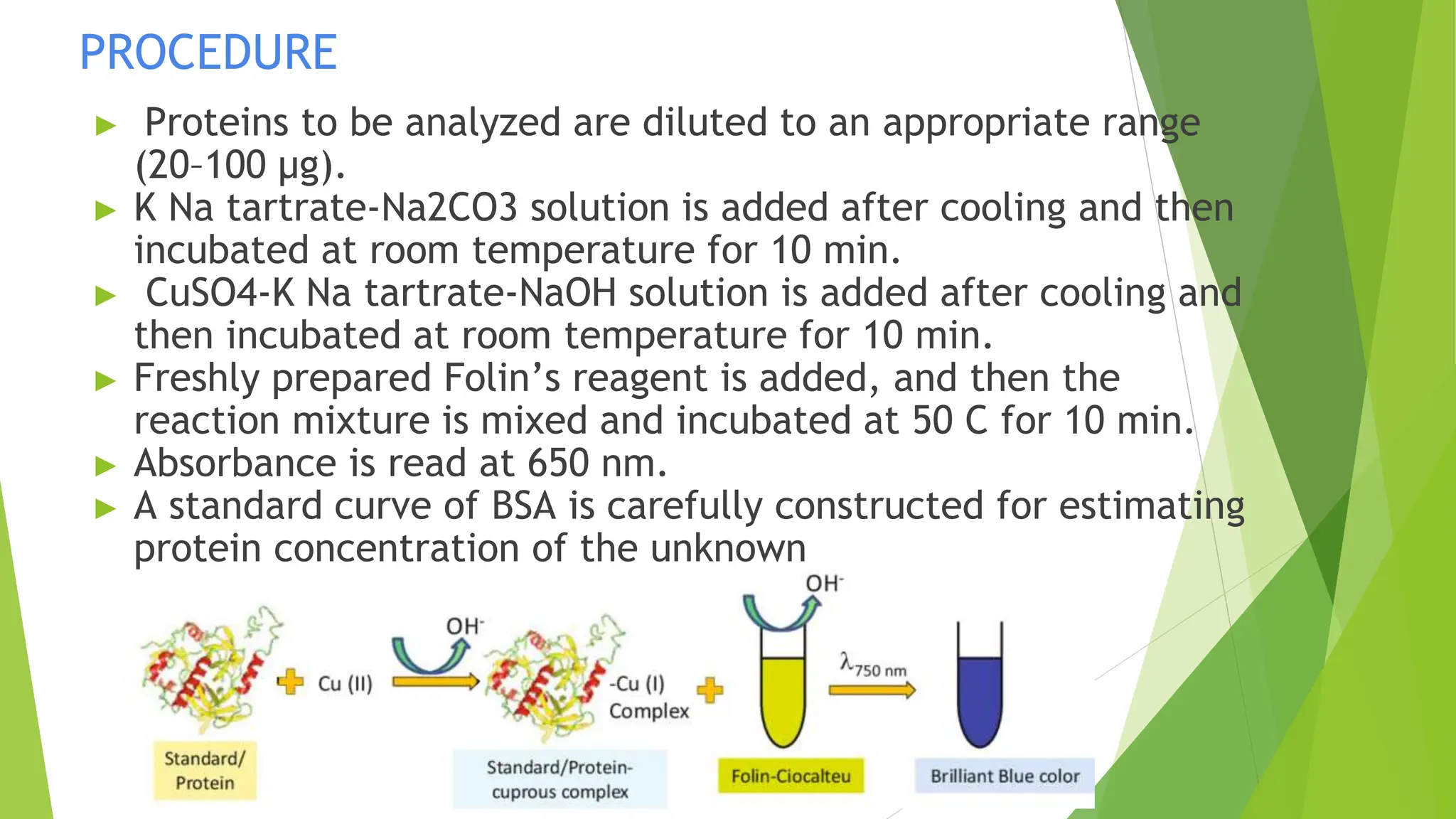
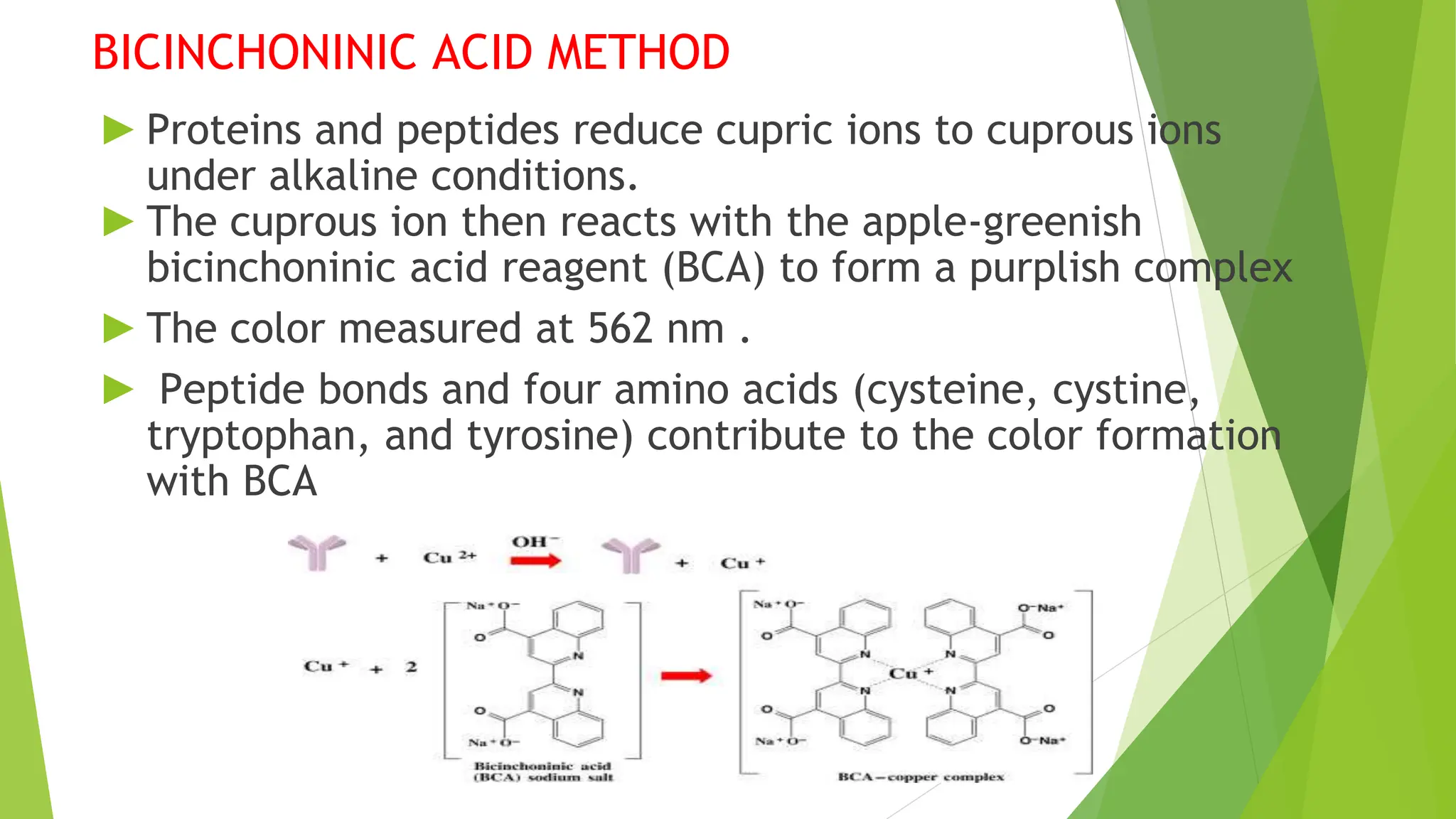
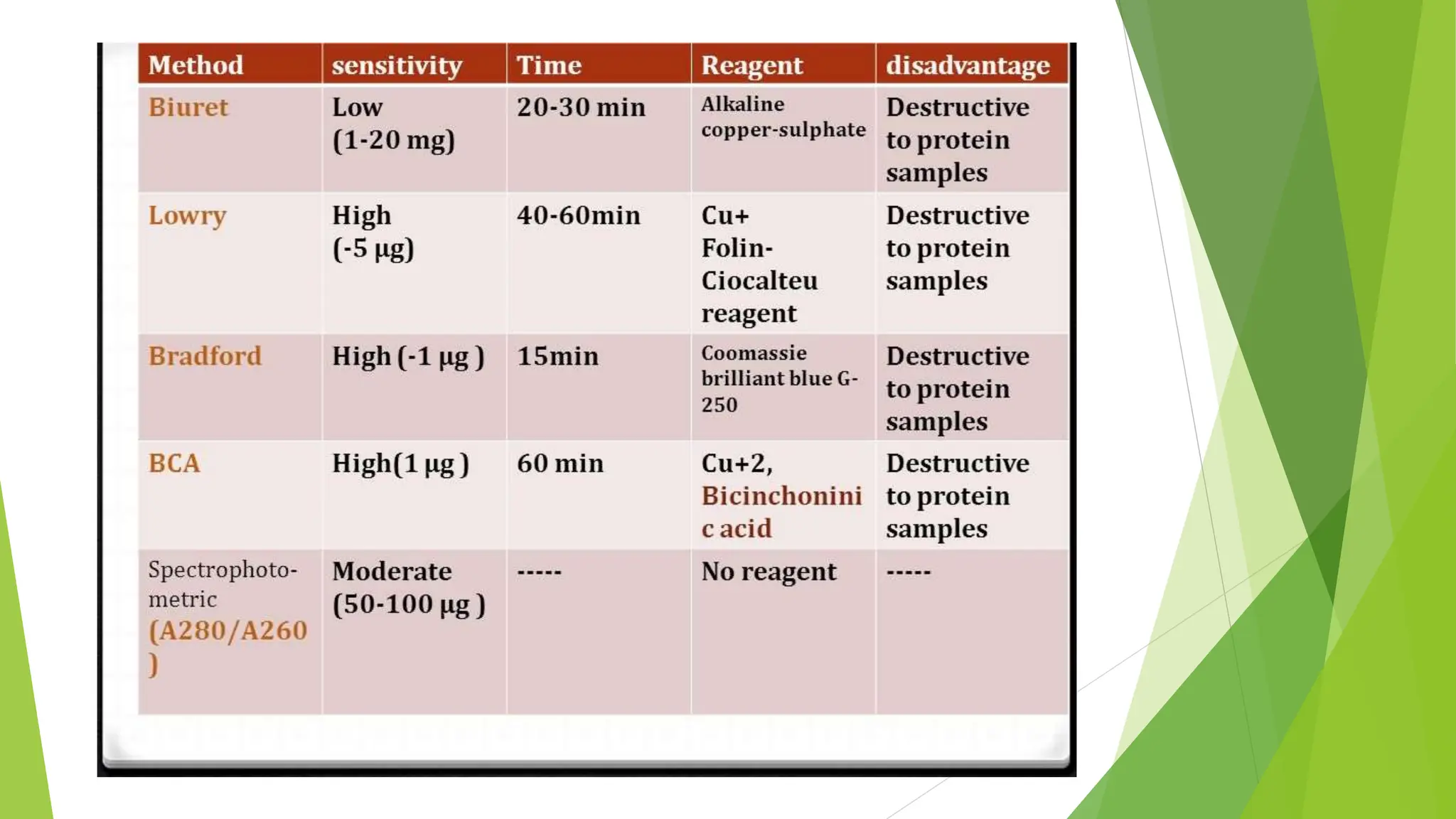
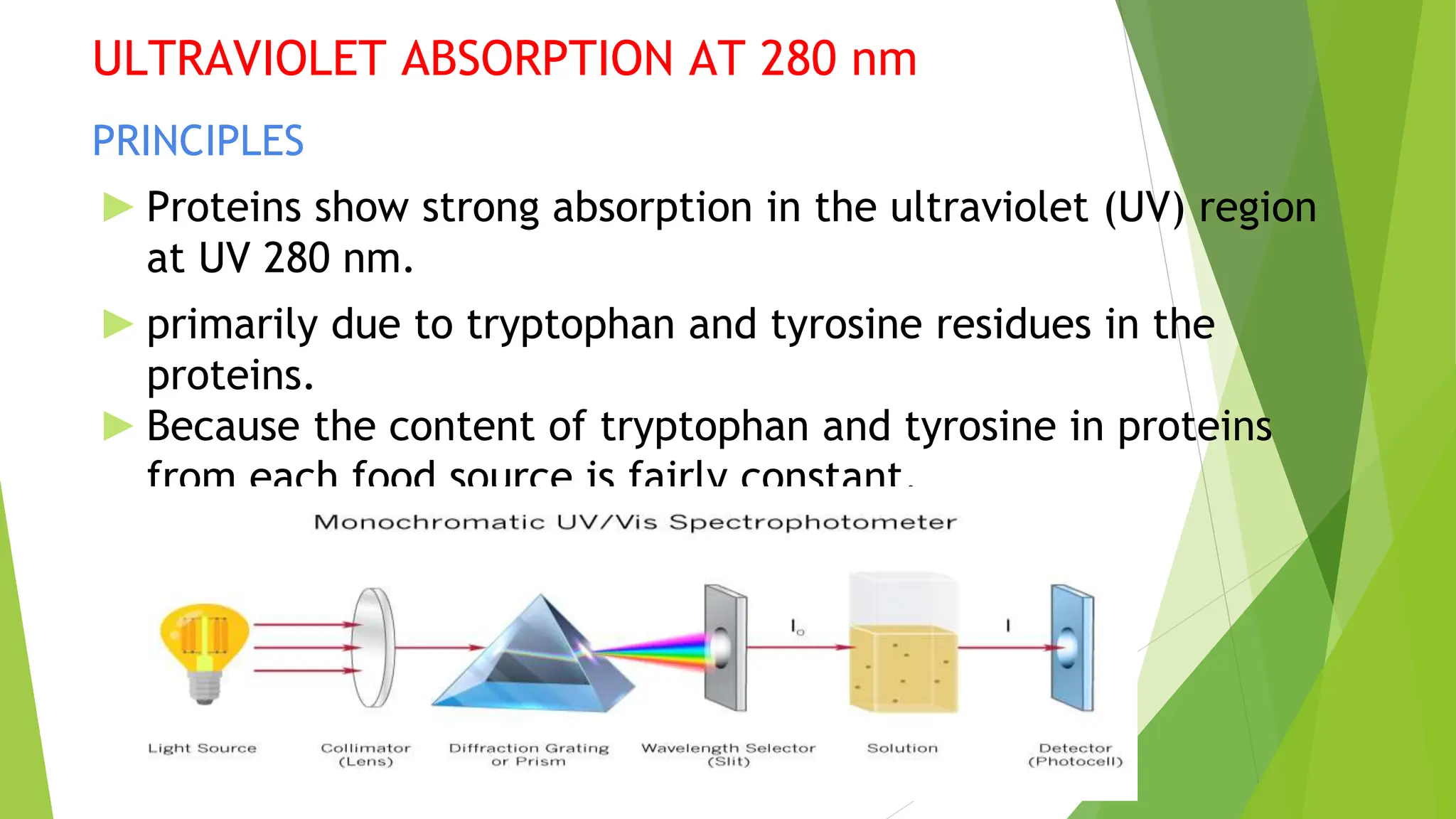
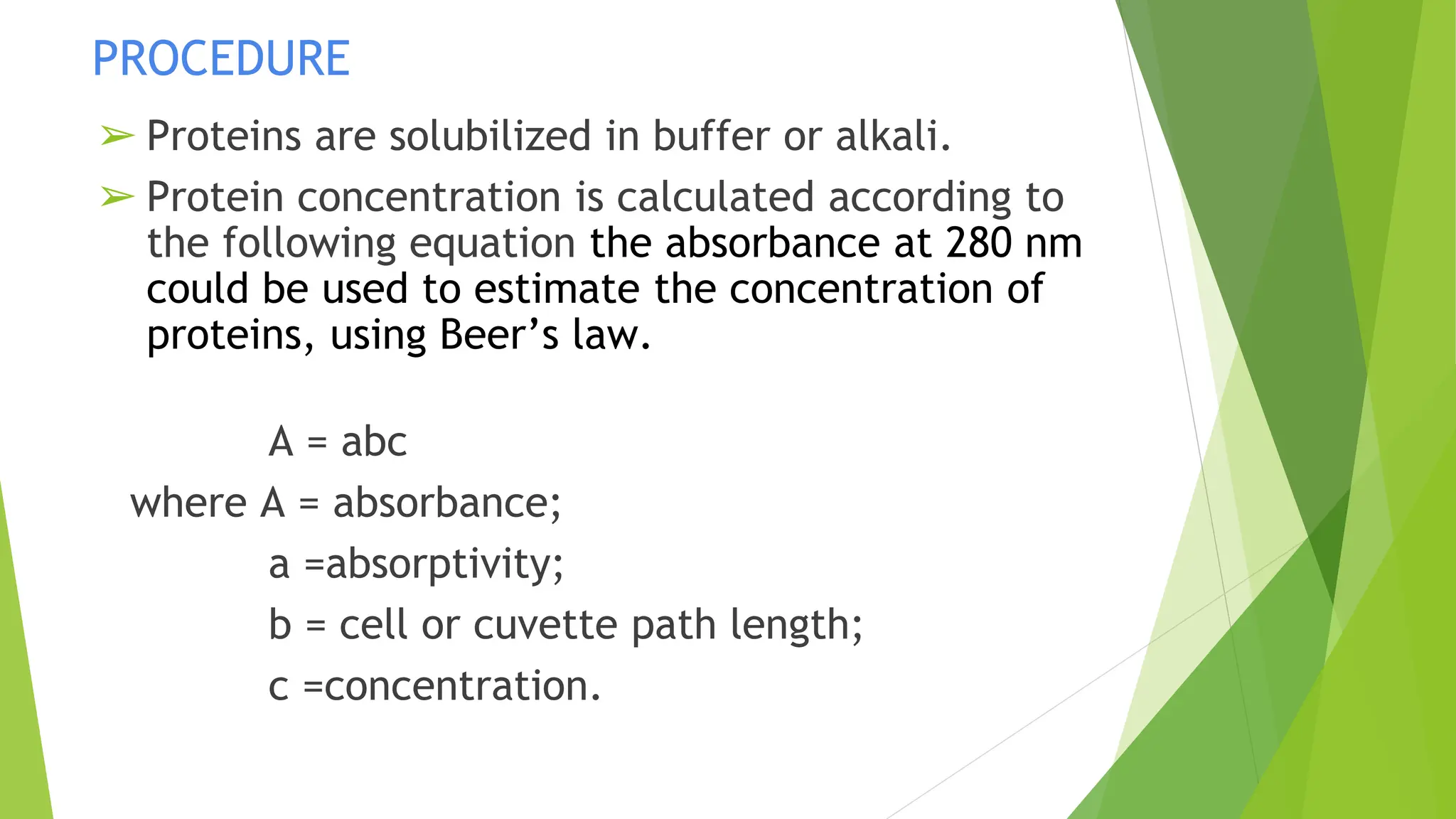
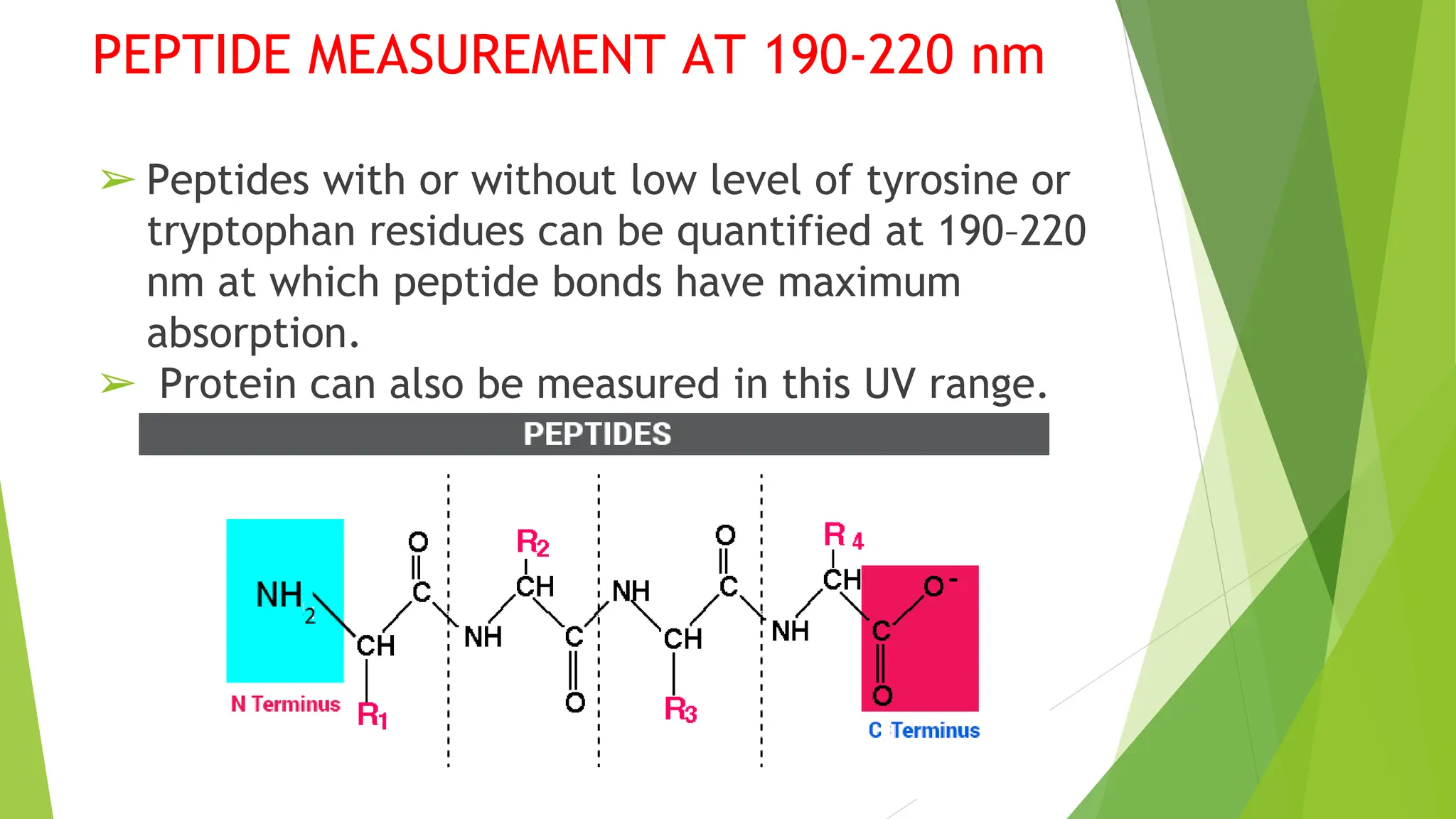
This document discusses several general methods for analyzing proteins, including the Kjeldahl method, Dumas method, infrared spectroscopy, colorimetric methods like dye-binding and Bradford's method, copper ion-based methods like Lowry and BCA, and ultraviolet absorption at 280nm. The Kjeldahl method involves digestion, neutralization and titration to determine protein content from nitrogen levels. The Dumas method uses combustion and gas chromatography. Infrared spectroscopy analyzes absorption of infrared radiation. Colorimetric methods exploit color changes from protein-dye complexes. Copper ion methods use biuret or phenol reactions. UV absorption at 280nm relies on tryptophan/tyrosine absorption.