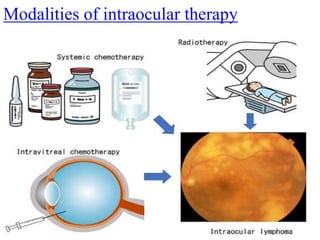
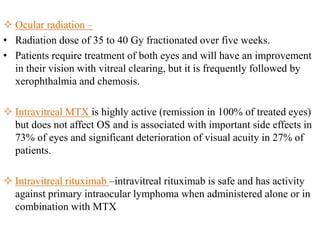
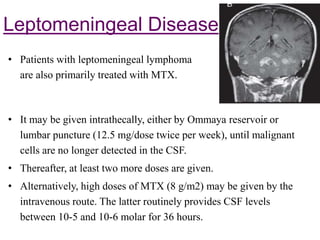
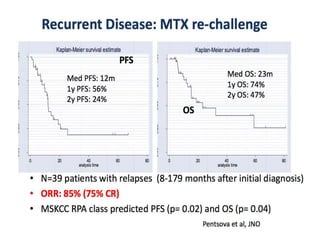
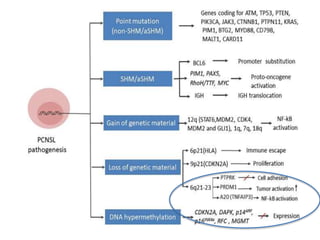
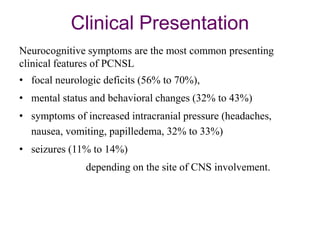
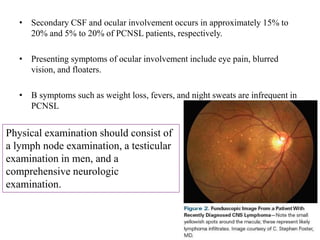
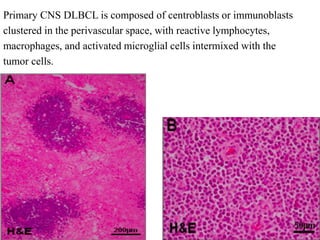
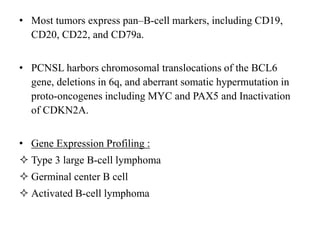
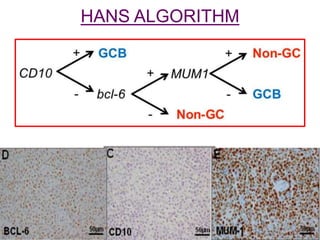
This document discusses primary central nervous system lymphoma (PCNSL). Some key points:
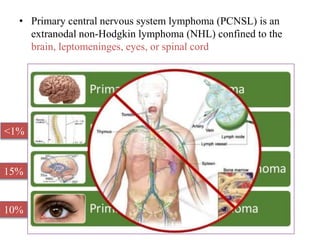
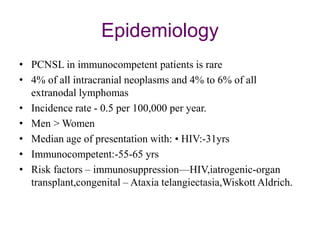
- PCNSL is a rare type of non-Hodgkin lymphoma confined to the brain, eyes, or spinal cord. It most commonly presents with neurocognitive symptoms in immunocompetent patients in their 50s-60s.
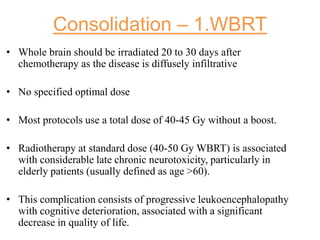
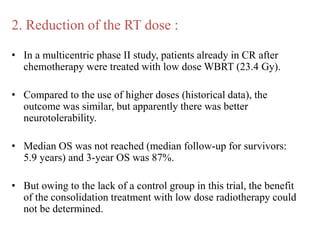
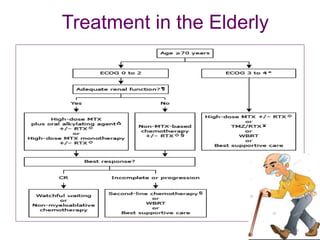
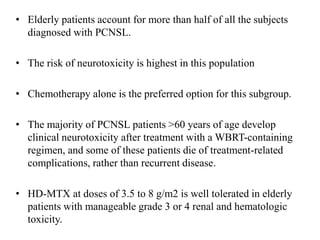
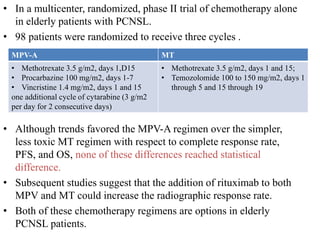
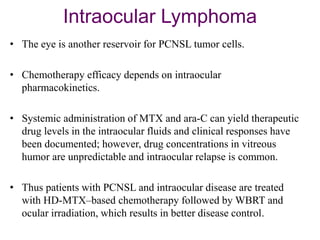
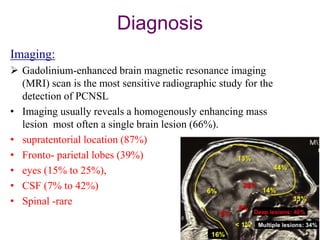
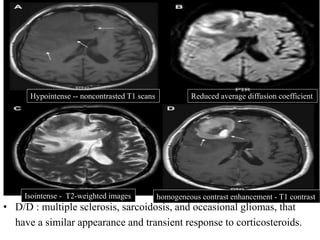
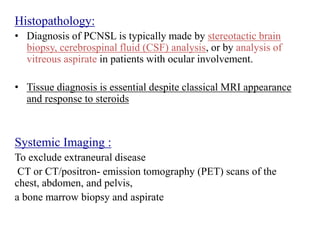
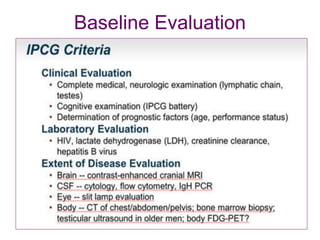
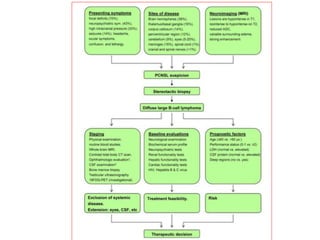
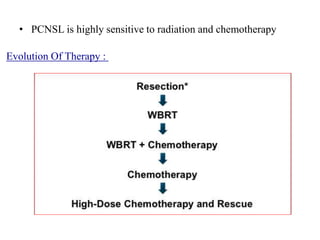
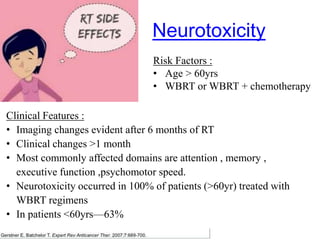
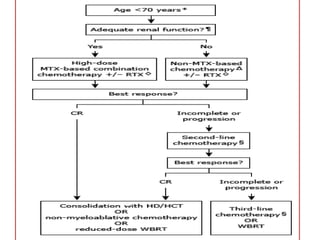
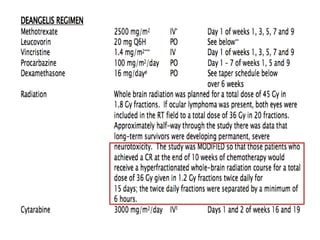
- Diagnosis involves brain imaging, biopsy, and ruling out systemic involvement. The standard treatment was whole brain radiation but this resulted in poor survival and neurotoxicity.
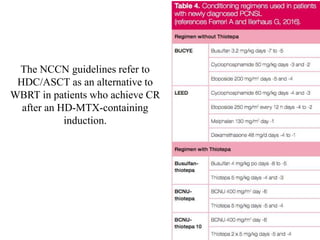
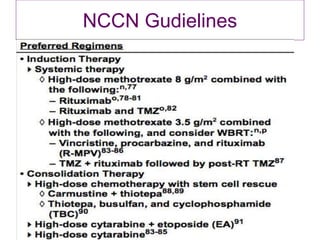
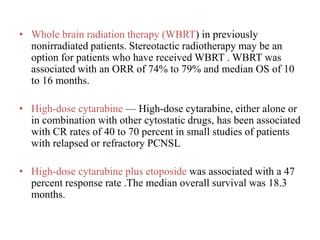
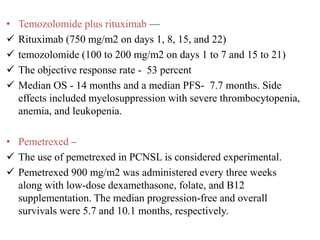
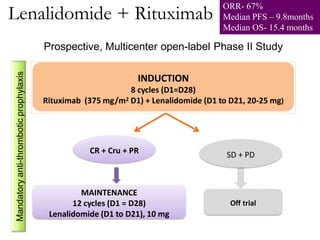
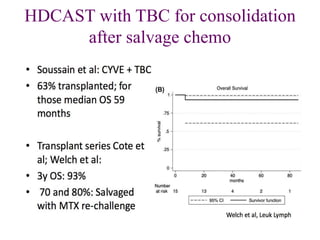
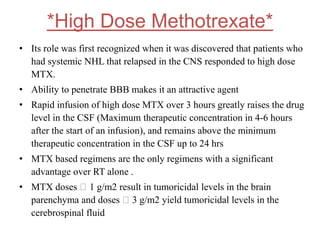
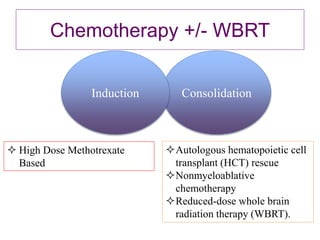
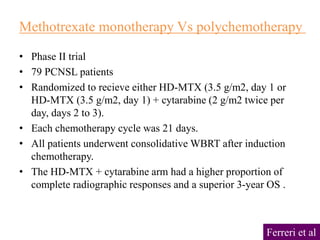
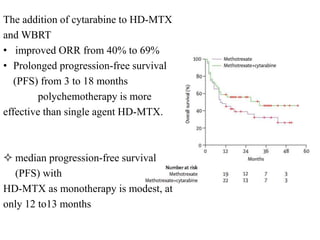
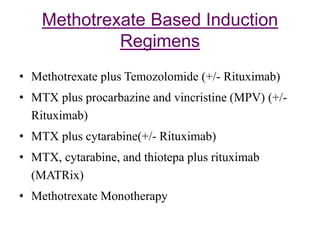
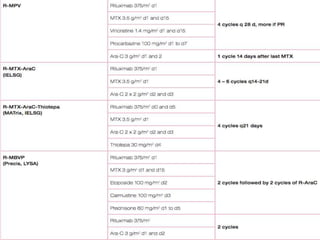
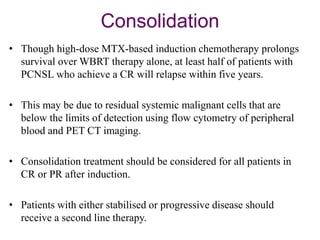
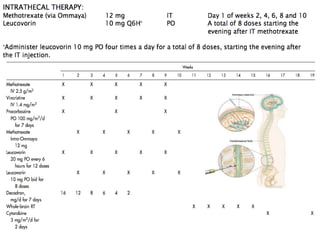
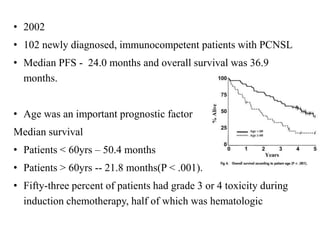
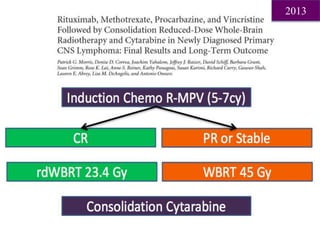
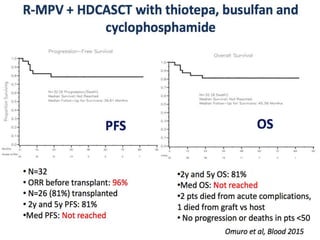
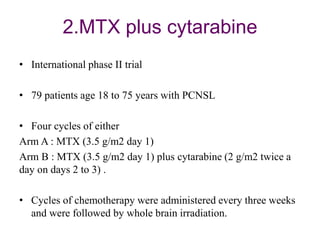
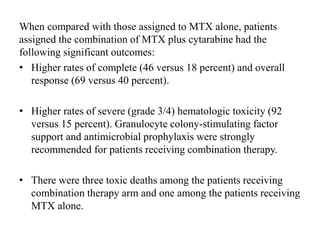
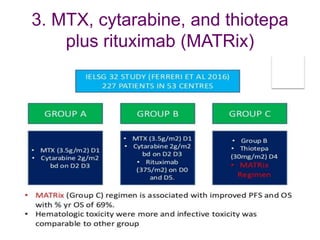
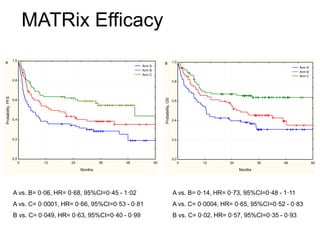
- Newer regimens combining high-dose methotrexate with other chemotherapy agents and reduced whole brain radiation have improved outcomes with median survival around 3 years and less neurotoxicity. Ongo










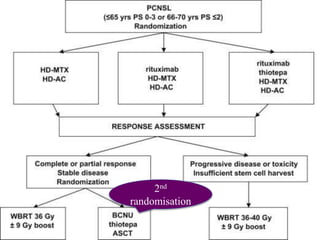
![Outcomes after
2nd randomization
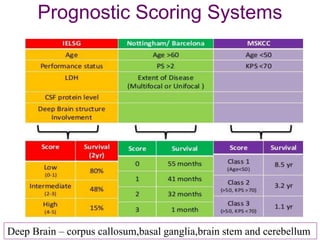
40 month follow up
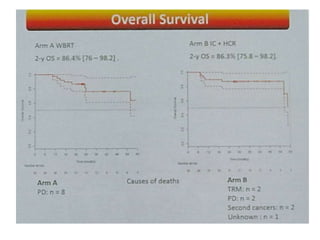
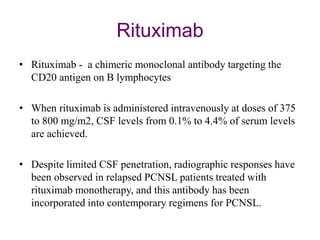
2-year PFS
• WBRT-- 80% (95% CI 70–90)
• ASCT -- 69% (59–79)
(hazard ratio [HR] 1·50, 95% CI 0·83–
2·71; p=0·17).
2-year OS
• WBRT - 85% (95% CI 75–95)
• ASCT - 71% (60–82)
(HR 1·67, 95% CI 0·86–3·23; p=0·12](https://image.slidesharecdn.com/primarycnslymphomamain-210518064857/85/Primary-cns-lymphoma-main-58-320.jpg)
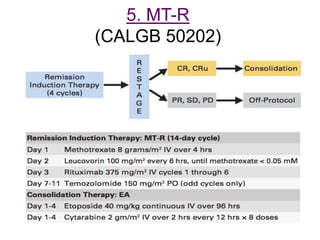
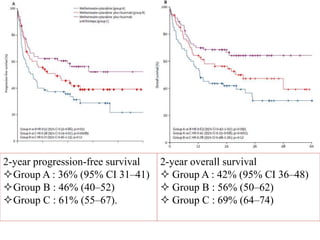
![Arm B: ASCT
Arm A: WBRT
Progression-free survival
2-y PFS = 86.8% [76.6 – 98.3]
2-y PFS =63.2% [49.5 – 80.5]
Arm A Arm B
Median FU 33.2 mois (24.3 - 89.7) 33.8 mois (23.7 - 64.5)](https://image.slidesharecdn.com/primarycnslymphomamain-210518064857/85/Primary-cns-lymphoma-main-62-320.jpg)