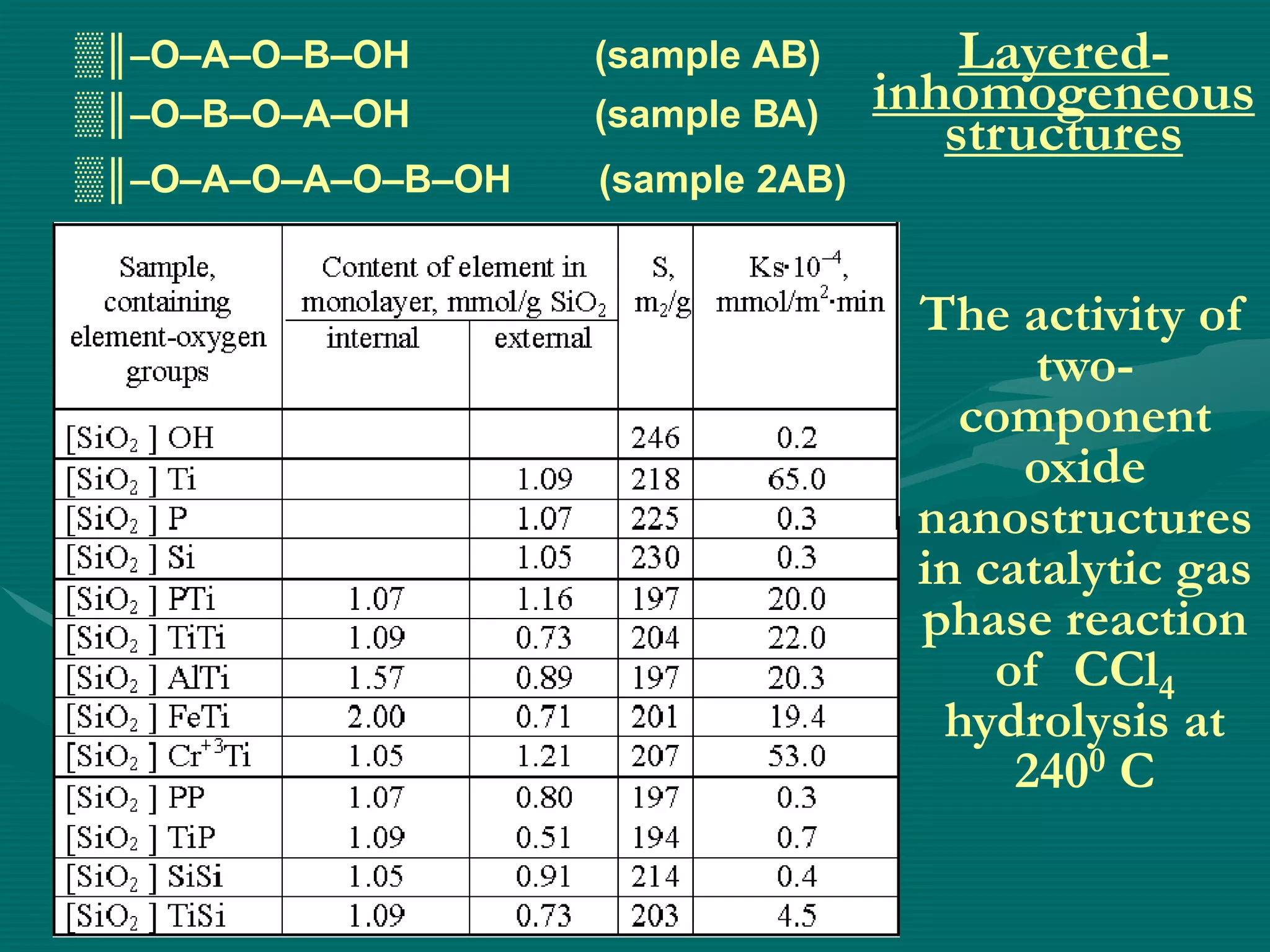
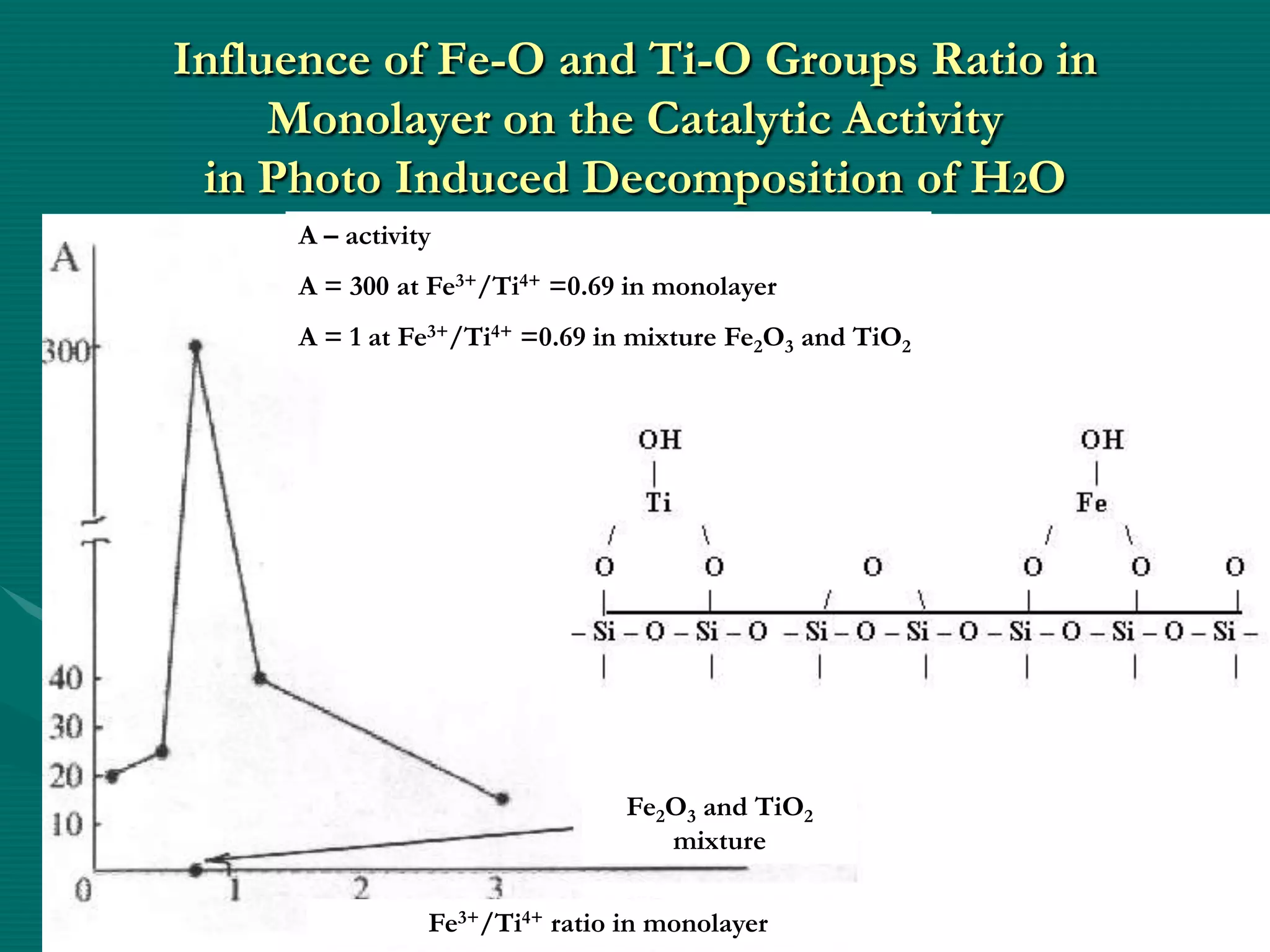
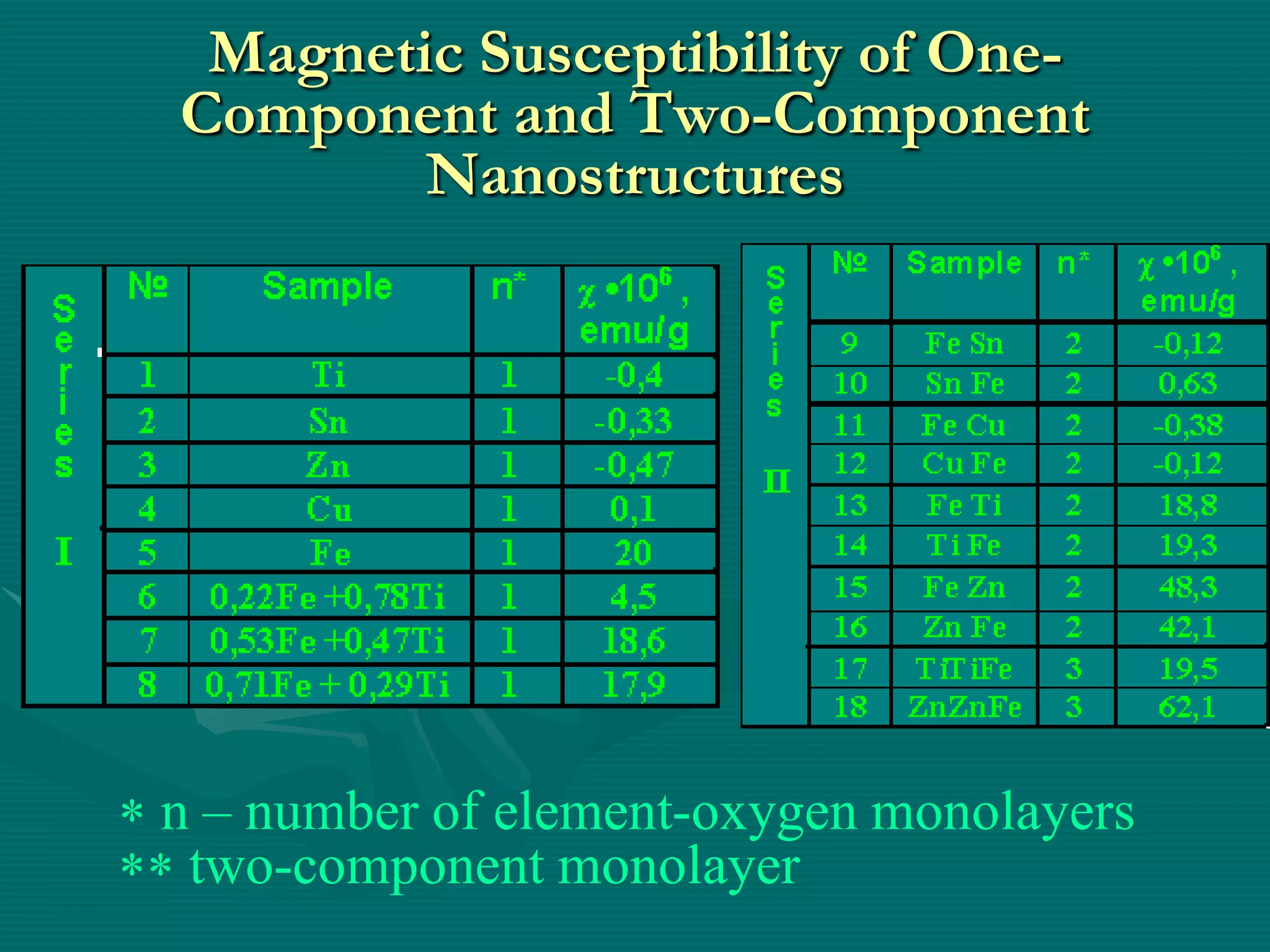
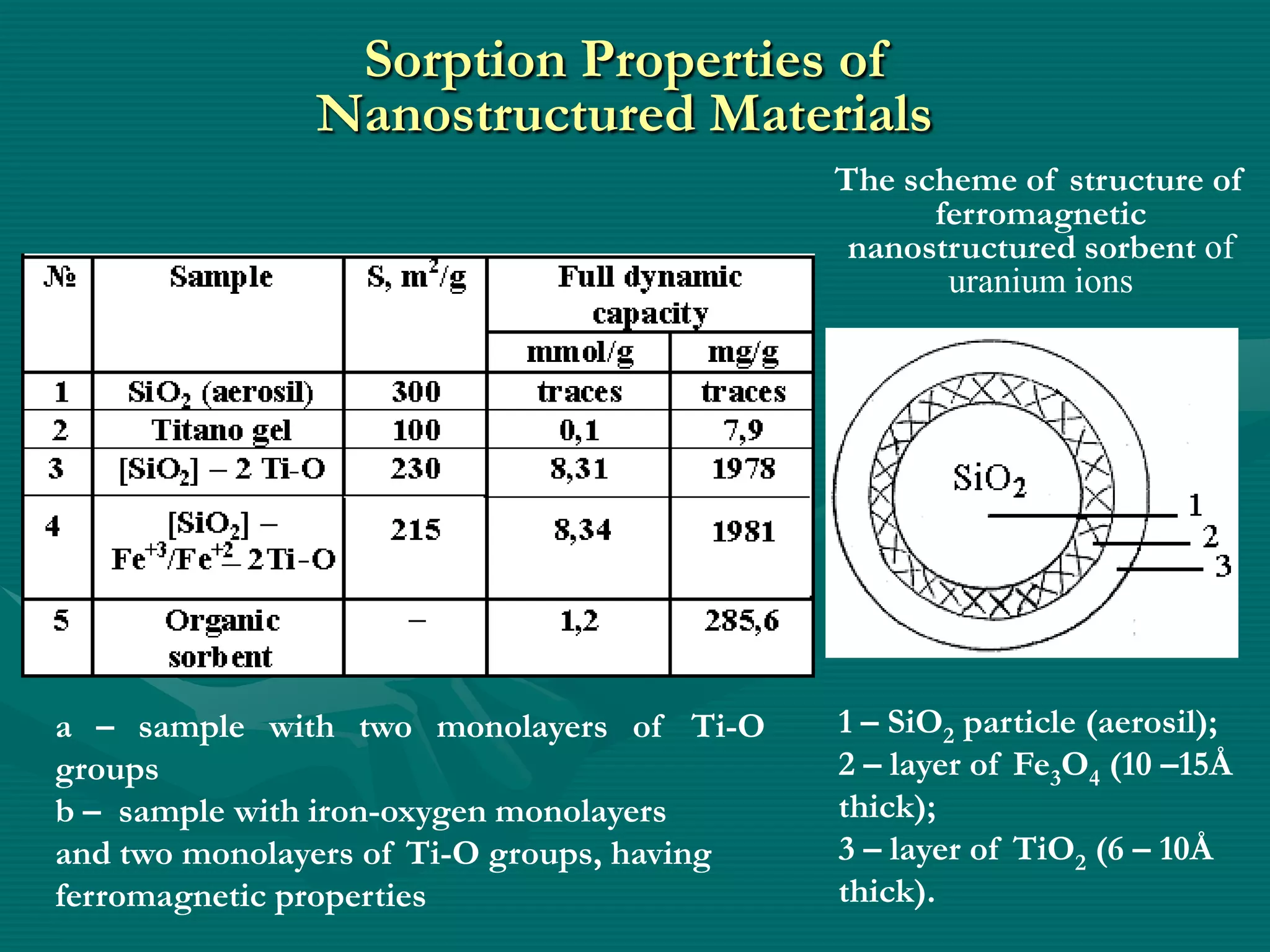
The document discusses nanostructured materials and their properties. It presents several schemes describing different types of two-dimensional nanostructures on silica surfaces based on homogeneous or periodic distribution of element-oxygen layers. It also examines the dimensional dependence of material properties on size, and how the ratio of chemical components in multi-layer nanostructures can influence properties like catalytic activity and magnetic susceptibility. Finally, it concludes that a scientific foundation now exists for designing highly organized nanostructured solids and materials by controlling composition and structure at the nanoscale.

![The State of Two-Dimensional
Nanostructures on the Silica Surface
• А – with homogeneous character of distribution of
chemical composition and state, for example, monolayers
of element-oxygen groups of the same chemical
composition, l – monolayer thickness, L – layer thickness,
l1 = l2 = l3 = l4; 1– for example, Ti–O monolayers.
• B – periodic distribution of element-oxygen layers
along z axis, (L – layer thickness) consisting of the certain
quantity of monolayers, for example, according to the
scheme: 1 –Fe-O groups, 2 –Ti-O groups (a – two-layer
group, l1 = l2 , б, в – four-layer group l1 = l3 и l2 = l4 ).
• C – aperiodic distribution of element-oxygen layers
along z axis, four-layer structure l1 l2 l3 l4
• D – aperiodic distribution of atoms on the plane of
surface monolayer, top view: х –Fe-O groups, о –Ti-O
groups; а, б – different ratios of groups Fe-O and Ti-O.
• E - aperiodic distribution of “zero-dimensional"
structures on the plane of support: 1 –Si , 2 – [ Fe]](https://image.slidesharecdn.com/prezenteng-120831060857-phpapp02/75/Prezent-eng-2-2048.jpg)
![Energetic Diagram of Reaction with Kinetic and
Thermodynamic Control
[SiO2]m-1O1,5SiOH + Cl2 [SiO2]m-1O1,5SiCl SiCl4
A B C
1 – reaction A → B
dominates, product
B is kinetically stable;
Free Energy
2 – side reaction A → C
dominates;
3 – reaction A → B
dominates, but the
product B is kinetically
unstable and converts
rapidly into C.
Reaction coordinate](https://image.slidesharecdn.com/prezenteng-120831060857-phpapp02/75/Prezent-eng-3-2048.jpg)
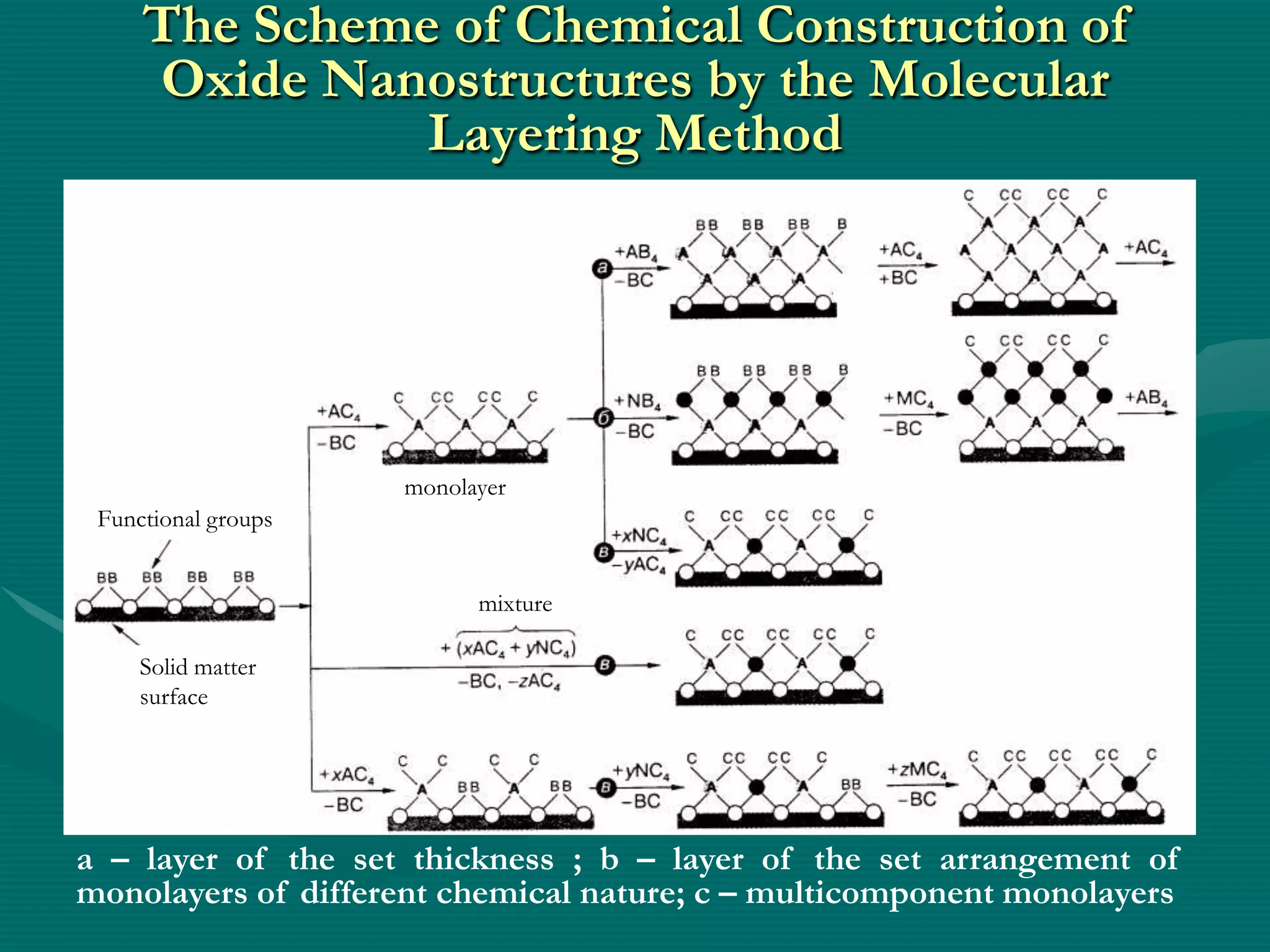
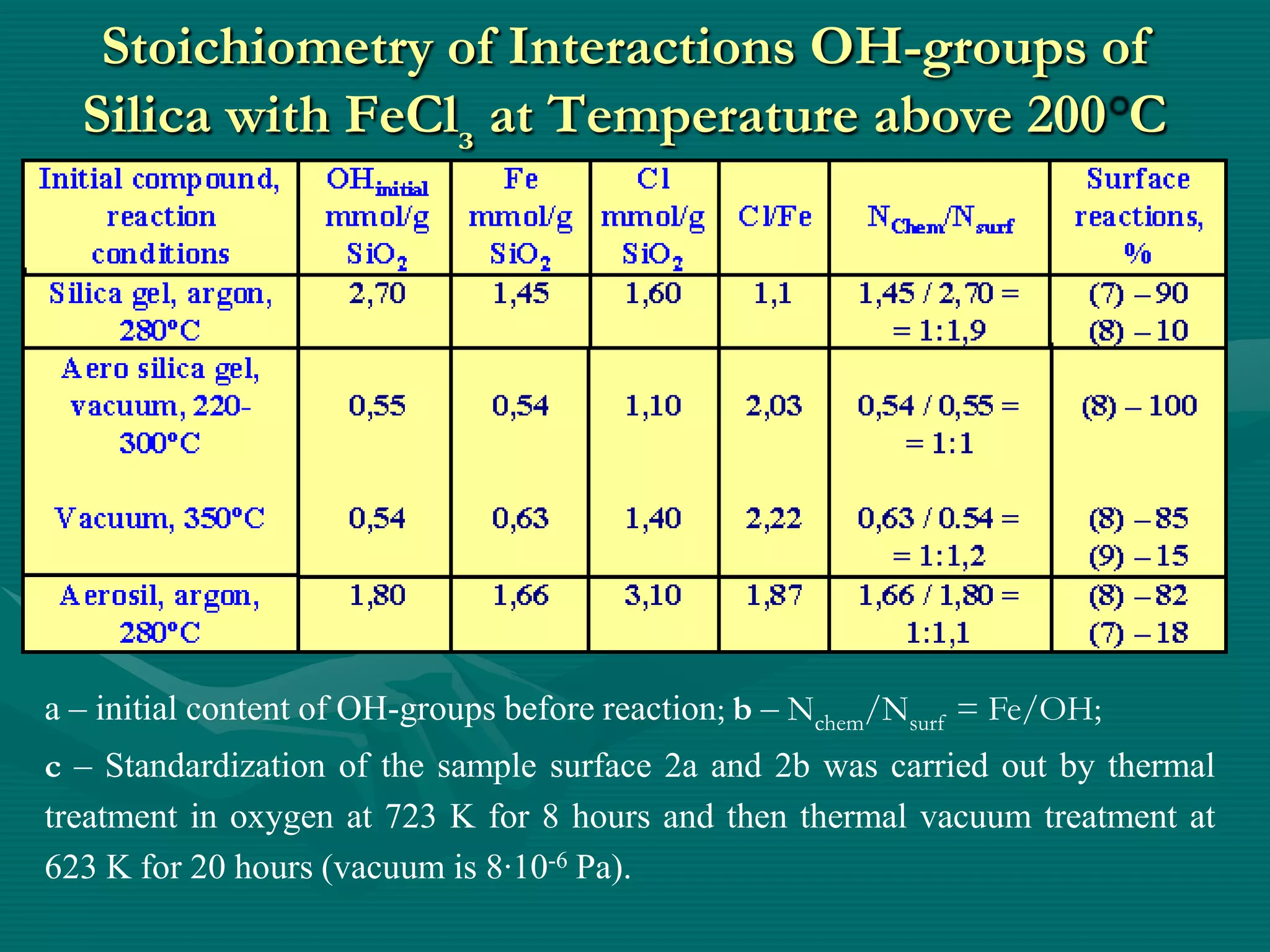
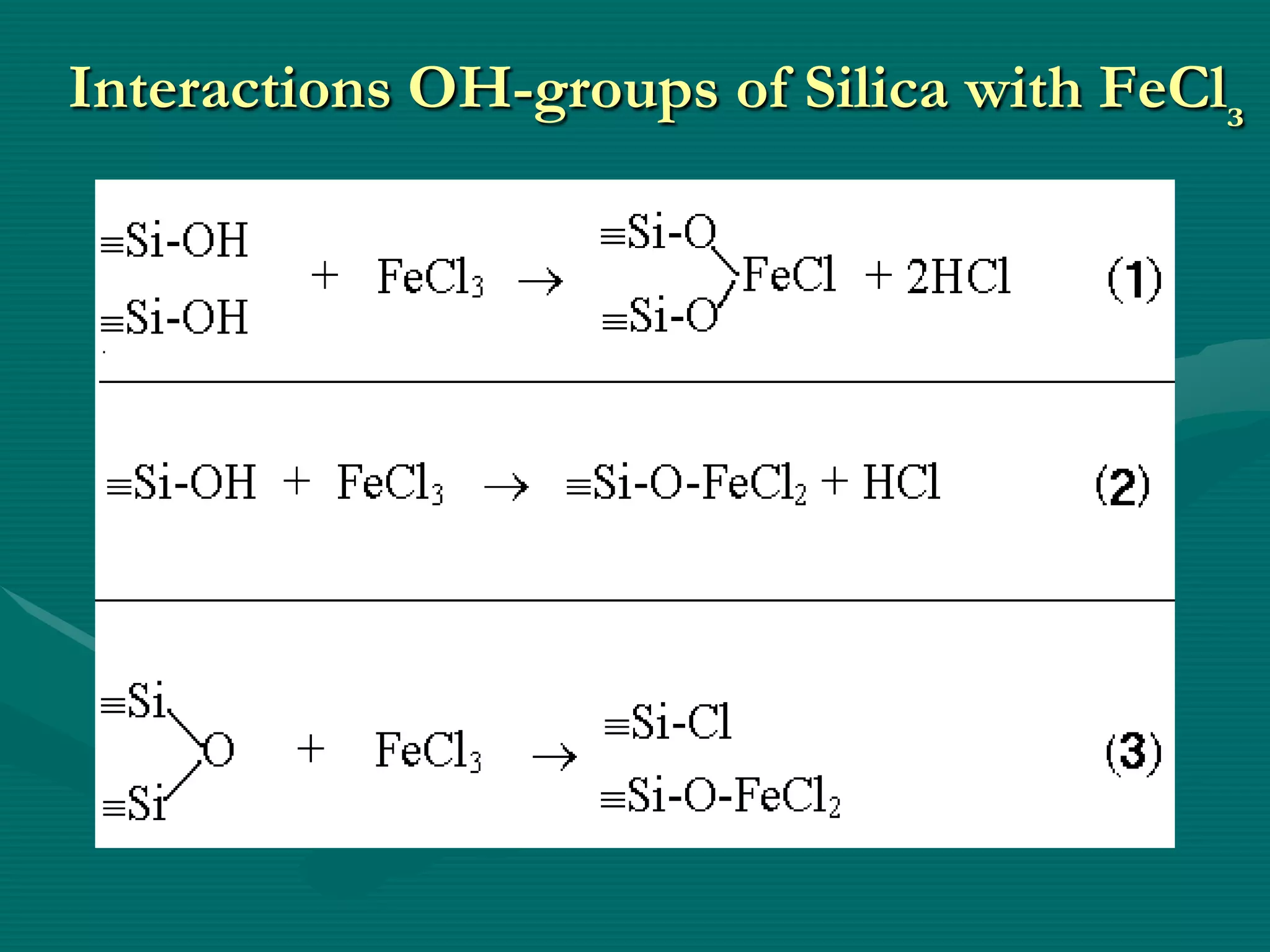
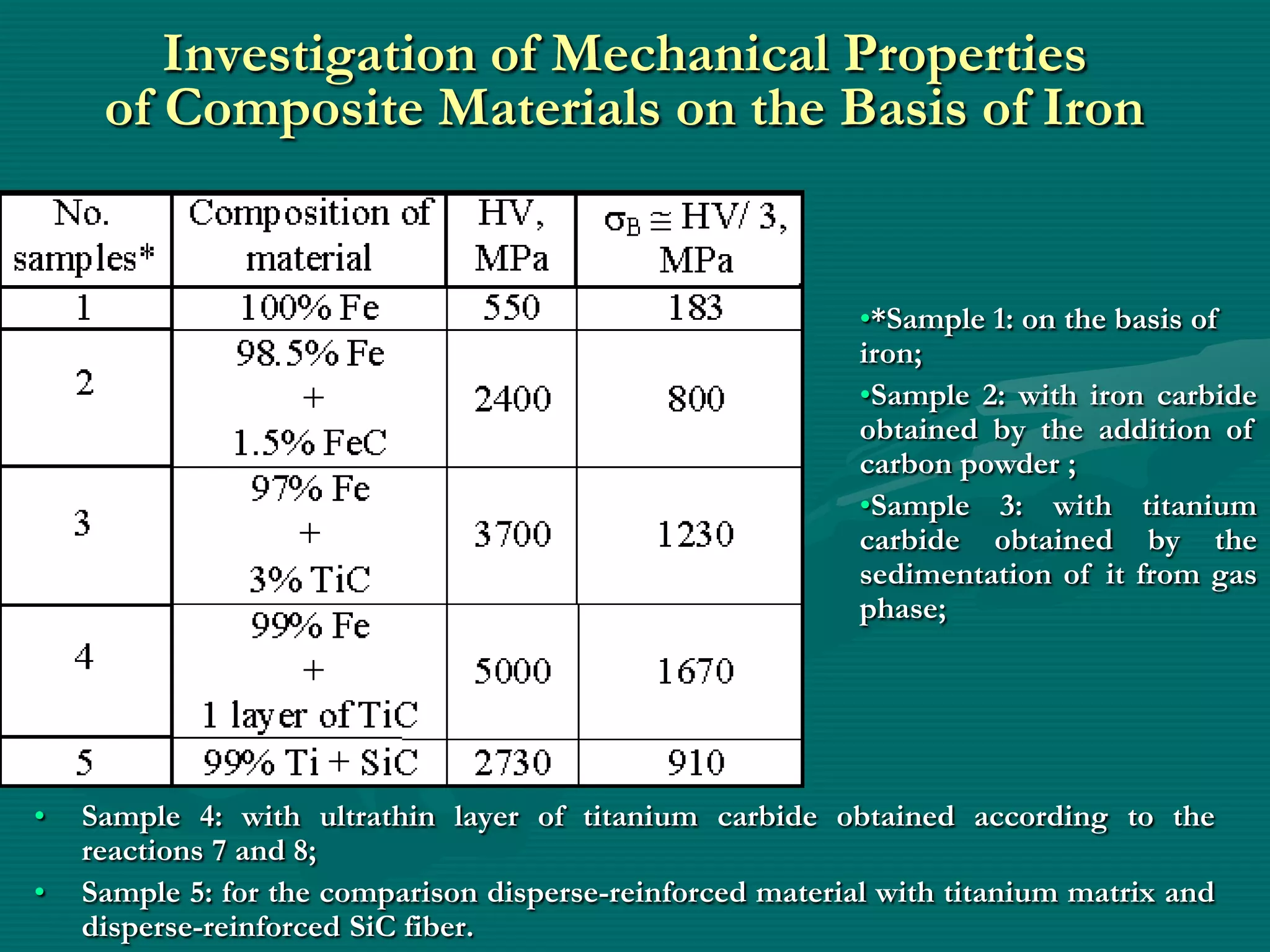
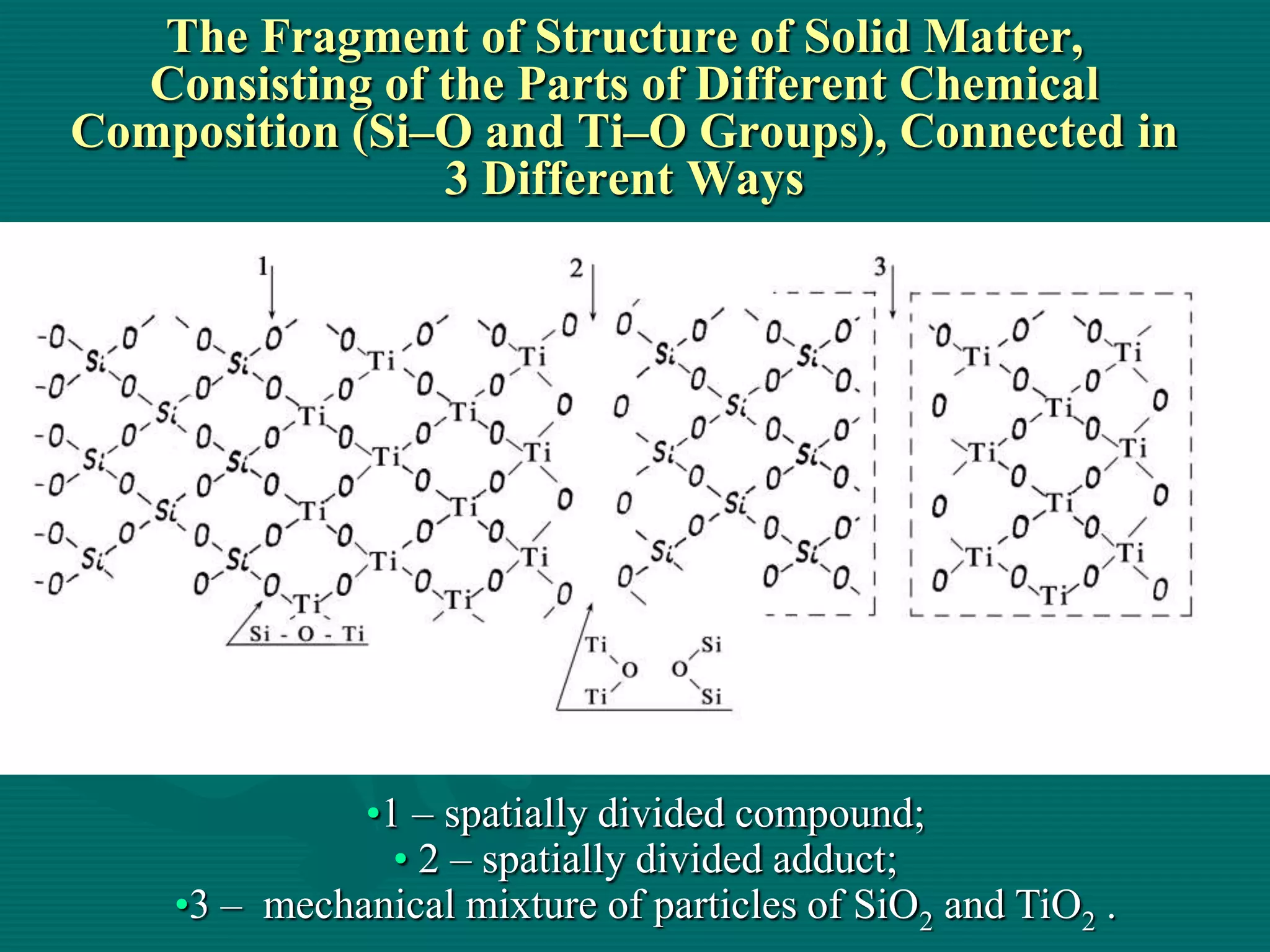
![The Scheme of State of
Solid Chemical Compounds
3
1 – initial metal
oxide (MOn);
2
2–surface chemical
compound [MO]n-1
1
(M– O)2 Si(OH)2 ;
3–spatially divided
compound [MOn]–
[SiO2];
1 – conditional
(internal) border of
division](https://image.slidesharecdn.com/prezenteng-120831060857-phpapp02/75/Prezent-eng-7-2048.jpg)
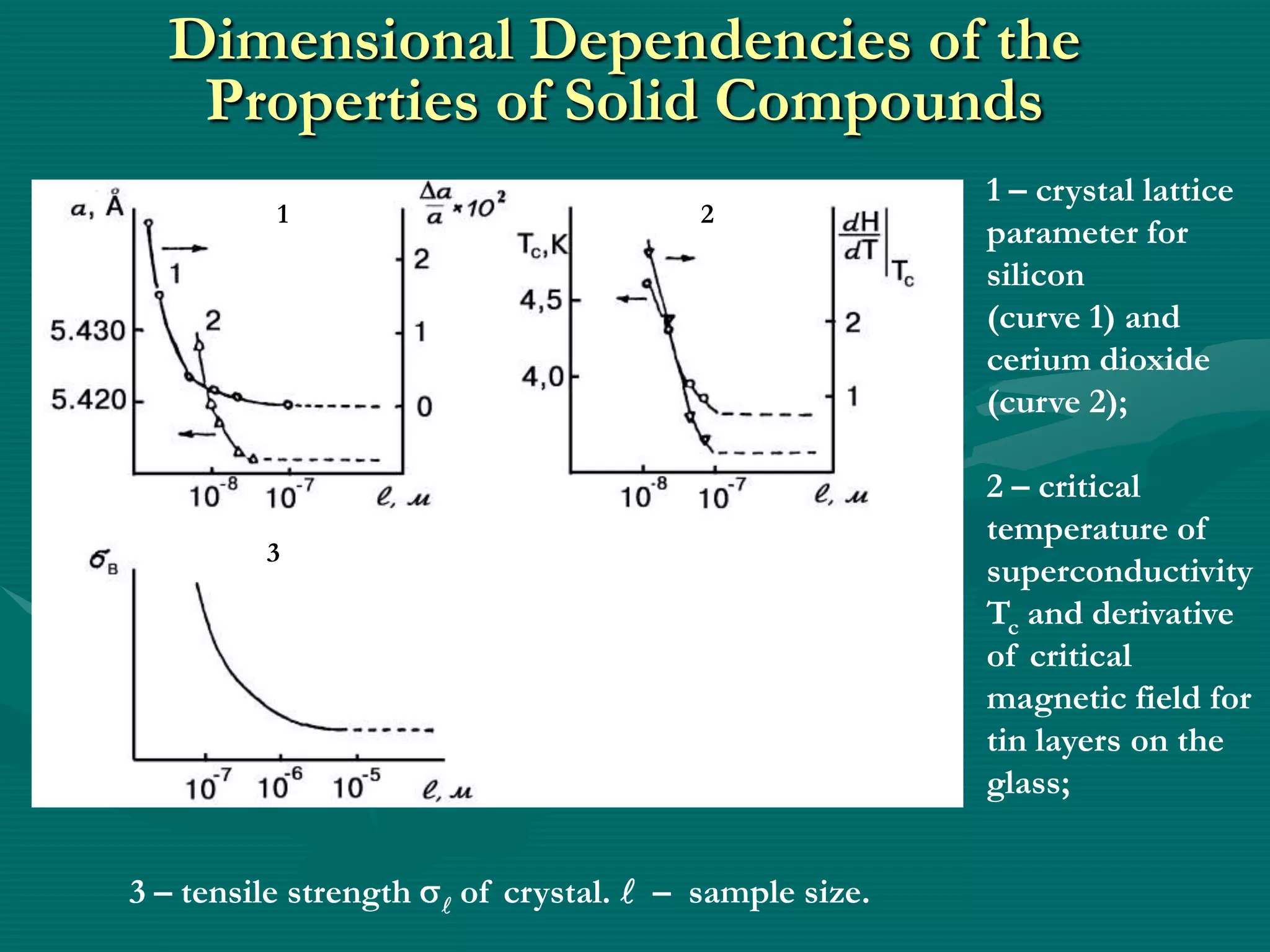
![Generalized View of Dependence of Properties
on Size of Chemical Matter
Subcrystal
Property
Massive
crystal a – for the solid matter;
b – change of the value of
specific Surface S depending on
Size of Solid Matter ()
b [according to V.B. Aleskovskii].
1 – size of minimal solid matter
(nanoobject);
2 – size of dispersed solid state
(micro object);
3 – massive (macroscopic) solid.](https://image.slidesharecdn.com/prezenteng-120831060857-phpapp02/75/Prezent-eng-10-2048.jpg)
![The State of Two-Dimensional
Nanostructures on the Silica Surface
• А – with homogeneous character of distribution of
chemical composition and state, for example, monolayers
of element-oxygen groups of the same chemical
composition, l – monolayer thickness, L – layer thickness,
l1 = l2 = l3 = l4; 1– for example, Ti–O monolayers.
• B – periodic distribution of element-oxygen layers
along z axis, (L – layer thickness) consisting of the certain
quantity of monolayers, for example, according to the
scheme: 1 –Fe-O groups, 2 –Ti-O groups (a – two-layer
group, l1 = l2 , б, в – four-layer group l1 = l3 и l2 = l4 ).
• C – aperiodic distribution of element-oxygen layers
along z axis, four-layer structure l1 l2 l3 l4
• D – aperiodic distribution of atoms on the plane of
surface monolayer, top view: х –Fe-O groups, о –Ti-O
groups; а, б – different ratios of groups Fe-O and Ti-O.
• E - aperiodic distribution of “zero-dimensional"
structures on the plane of support: 1 –Si , 2 – [ Fe]](https://image.slidesharecdn.com/prezenteng-120831060857-phpapp02/75/Prezent-eng-11-2048.jpg)