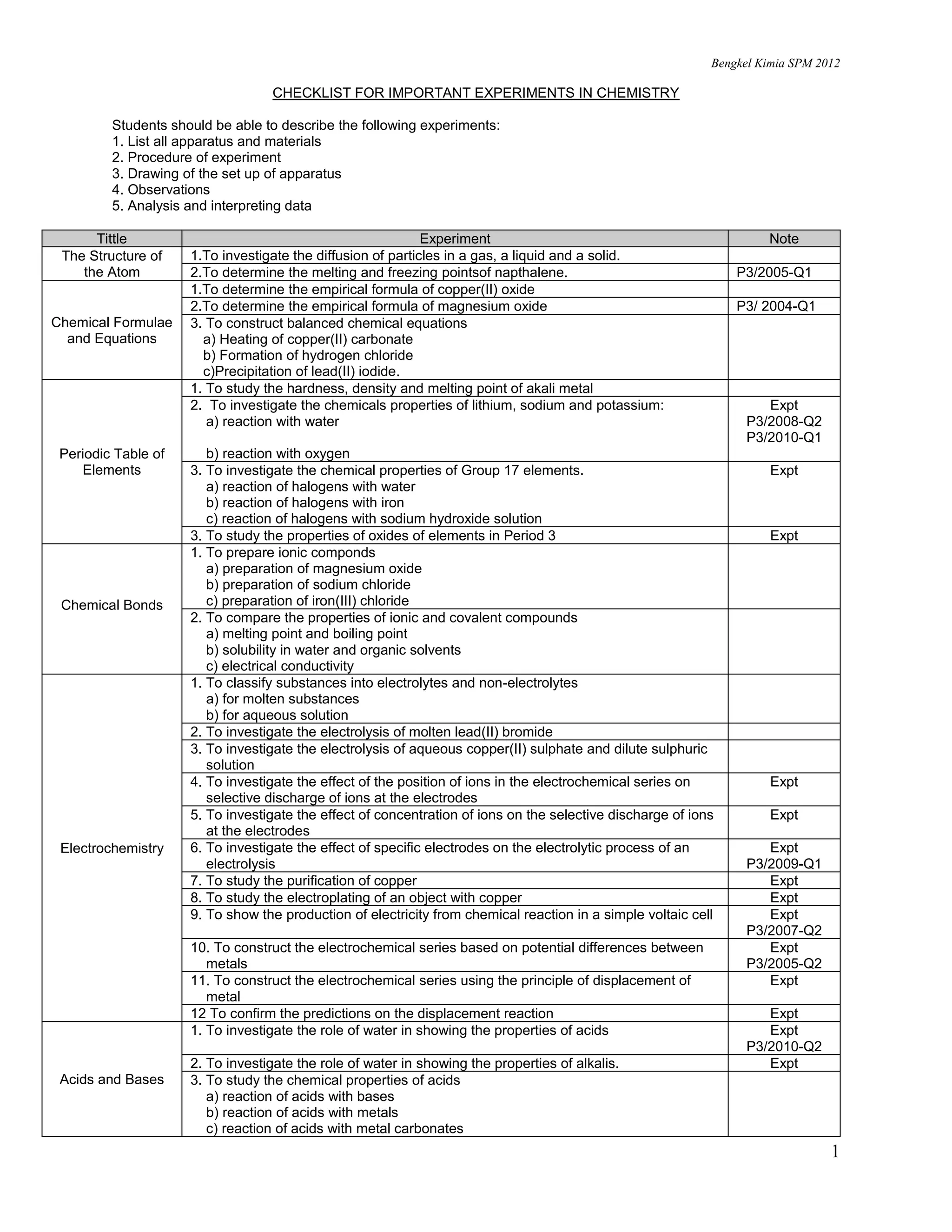
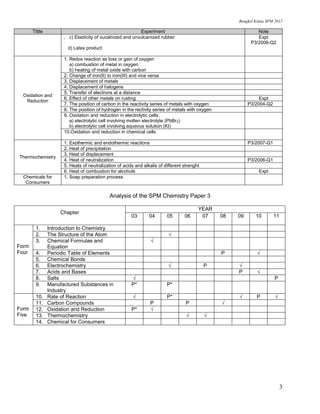
This document provides a checklist of important experiments in chemistry for SPM (Sijil Pelajaran Malaysia) students. It lists 14 chemistry topics and the key experiments students should be able to describe for each topic. These include experiments on the structure of the atom, chemical formulae and equations, the periodic table of elements, electrochemistry, acids and bases, salts, rate of reaction, carbon compounds, oxidation and reduction, and thermochemistry. For each experiment, students are expected to describe the apparatus, materials, procedure, observations, analysis, and interpreting data. The document also analyzes which chemistry topics were examined in SPM Paper 3 from 2003 to 2011.